Conjugate Nucleophilic Addition to α,β‑Unsaturated Aldehydes and Ketones | Chemistry Optional Notes for UPSC PDF Download
| Table of contents |

|
| Conjugate Nucleophilic Addition |

|
| Conjugate Addition of Amines |

|
| Conjugate Addition of Water |

|
| Conjugate Addition of Alkyl Groups: Organocopper Reactions |

|
| Solved Example |

|
Conjugate Nucleophilic Addition
All the reactions we’ve been discussing to this point have involved the addition of a nucleophile directly to the carbonyl group, a so-called 1,2-addition. Closely related to this direct addition is the conjugate addition, or 1,4-addition, of a nucleophile.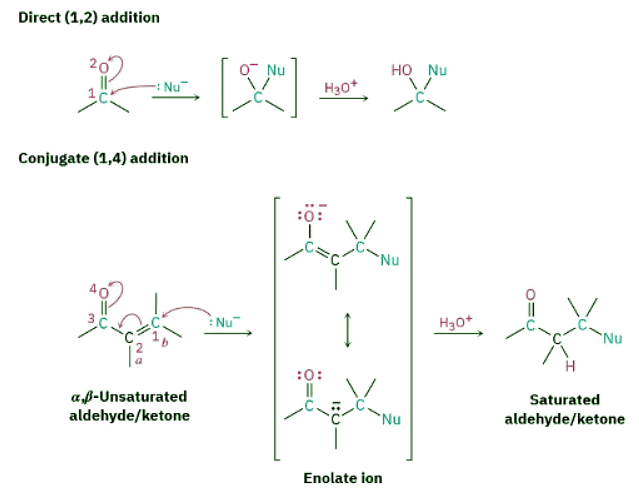
Figure 19.14: A comparison of direct (1,2) and conjugate (1,4) nucleophilic addition reactions. In conjugate addition, a nucleophile adds to the β carbon of an α,β-unsaturated aldehyde or ketone and protonation occurs on the α carbon.
The conjugate addition of a nucleophile to an α,β-unsaturated aldehyde or ketone is caused by the same electronic factors that are responsible for direct addition. The electronegative oxygen atom of the α,β-unsaturated carbonyl compound withdraws electrons from the β carbon, thereby making it electron-poor and more electrophilic than a typical alkene carbon.
As noted, conjugate addition of a nucleophile to the β carbon of an α,β-unsaturated aldehyde or ketone leads to an enolate ion intermediate, which is protonated on the α carbon to give the saturated product (Figure 19.14). The net effect is addition of the nucleophile to the C═C bond, with the carbonyl group itself unchanged. In fact, of course, the carbonyl group is crucial to the success of the reaction. Without the carbonyl group, the C═C bond would not be activated for addition, and no reaction would occur.
Conjugate Addition of Amines
Both primary and secondary amines add to α,β-unsaturated aldehydes and ketones to yield β-amino aldehydes and ketones rather than the alternative imines. Under typical reaction conditions, both modes of addition occur rapidly. But because the reactions are reversible, they generally proceed with thermodynamic control rather than kinetic control, so the more stable conjugate addition product is often obtained with the complete exclusion of the less stable direct addition product.
Conjugate Addition of Water
Water can add reversibly to α,β-unsaturated aldehydes and ketones to yield β-hydroxy aldehydes and ketones, although the position of the equilibrium generally favors unsaturated reactant rather than saturated adduct. Related additions to α,β-unsaturated carboxylic acids occur in numerous biological pathways, such as the citric acid cycle of food metabolism in which cis-aconitate is converted into isocitrate by conjugate addition of water to a double bond.

Conjugate Addition of Alkyl Groups: Organocopper Reactions
The conjugate addition of an alkyl or other organic group to an α,β-unsaturated ketone (but not aldehyde) is one of the more useful 1,4-addition reactions, just as direct addition of a Grignard reagent is one of the more useful 1,2-additions.
Conjugate addition of an organic group is carried out by treating the α,β-unsaturated ketone with a lithium diorganocopper reagent, R2CuLi. Lithium diorganocopper (Gilman) reagents are prepared by reaction between 1 equivalent of copper(I) iodide and 2 equivalents of an organolithium regent, RLi. The organolithium reagent, in turn, is formed by reaction of lithium metal with an organohalide in the same way that a Grignard reagent is prepared by reaction of magnesium metal with an organohalide.
Primary, secondary, and even tertiary alkyl groups undergo the conjugate addition reaction, as do aryl and alkenyl groups. Alkynyl groups, however, react poorly in the conjugate addition process. Diorganocopper reagents are unique in their ability to give conjugate addition products. Other organometallic reagents, such as Grignard reagents and organolithiums, normally result in direct carbonyl addition on reaction with α,β-unsaturated ketones.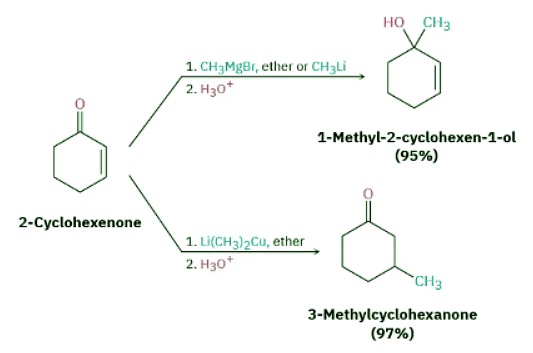
The mechanism of this reaction is thought to involve conjugate nucleophilic addition of the diorganocopper anion, R2Cu–, to the unsaturated ketone to give a copper-containing intermediate. Transfer of an R group from copper to carbon, followed by elimination of a neutral organocopper species, RCu, gives the final product.
Solved Example
Example: Using a Conjugate Addition Reaction
How might you use a conjugate addition reaction to prepare 2-methyl-3-propylcyclopentanone? Ans: Strategy: A ketone with a substituent group in its β position might be prepared by a conjugate addition of that group to an α,β-unsaturated ketone. In the present instance, the target molecule has a propyl substituent on the β carbon and might therefore be prepared from 2-methyl-2-cyclopentenone by reaction with lithium dipropylcopper.
Ans: Strategy: A ketone with a substituent group in its β position might be prepared by a conjugate addition of that group to an α,β-unsaturated ketone. In the present instance, the target molecule has a propyl substituent on the β carbon and might therefore be prepared from 2-methyl-2-cyclopentenone by reaction with lithium dipropylcopper.
Solution:
FAQs on Conjugate Nucleophilic Addition to α,β‑Unsaturated Aldehydes and Ketones - Chemistry Optional Notes for UPSC
| 1. What is nucleophilic addition? |  |
| 2. How does conjugate addition of amines occur? |  |
| 3. What happens during the conjugate addition of water? |  |
| 4. How do alkyl groups participate in conjugate addition reactions with organocopper compounds? |  |
| 5. Can you provide an example of conjugate nucleophilic addition to α,β-unsaturated aldehydes and ketones? |  |

|
Explore Courses for UPSC exam
|

|
















