UPSC Exam > UPSC Notes > Chemistry Optional Notes for UPSC > Diels-Alder Cycloaddition Rxn & its Stereochemistry
Diels-Alder Cycloaddition Rxn & its Stereochemistry | Chemistry Optional Notes for UPSC PDF Download
The Diels-Alder (4+2) Cycloaddition Reaction
- A cycloaddition reaction is the concerted bonding together of two independent pi-electron systems to form a new ring of atoms. When this occurs, two pi-bonds are converted to two sigma-bonds, the simplest example being the hypothetical combination of two ethene molecules to give cyclobutane. This does not occur under normal conditions, but the cycloaddition of 1,3-butadiene to cyanoethene (acrylonitrile) does, and this is an example of the Diels-Alder reaction. The following diagram illustrates two cycloadditions, and introduces several terms that are useful in discussing reactions of this kind.

- In the hypothetical ethylene dimerization on the left, each reactant molecule has a pi-bond (colored orange) occupied by two electrons. The cycloaddition converts these pi-bonds into new sigma-bonds (colored green), and this transformation is then designated a [2+2] cycloaddition, to enumerate the reactant pi-electrons that change their bonding location.
- The Diels-Alder reaction is an important and widely used method for making six-membered rings, as shown on the right. The reactants used in such reactions are a conjugated diene, simply referred to as the diene, and a double or triple bond co-reactant called the dienophile, because it combines with (has an affinity for) the diene. The Diels-Alder cycloaddition is classified as a [4+2] process because the diene has four pi-electrons that shift position in the reaction and the dienophile has two.
Diels-Alder Mechanism
- The Diels-Alder reaction is a single step process, so the diene component must adopt an s-cisconformation in order for the end carbon atoms (#1 & #4) to bond simultaneously to the dienophile. For many acyclic dienes the s-trans conformer is more stable than the s-cis conformer (due to steric crowding of the end groups), but the two are generally in rapid equilibrium, permitting the use of all but the most hindered dienes as reactants in Diels-Alder reactions. In order for a Diels-Alder reaction to occur, the diene molecule must adopt what is called the s-cis conformation:

- In its usual form, the diene component is electron rich, and the best dienophiles are electron poor due to electron withdrawing substituents such as CN, C=O & NO2. The initial bonding interaction reflects this electron imbalance, with the two new sigma-bonds being formed simultaneously, but not necessarily at equal rates. Essentially, this process involves overlap of the 2p orbitals on carbons 1 and 4 of the diene with the two 2p orbitals on the sp2-hybridized carbons of the dienophile. Both of these new overlaps form new sigma bonds, and a new pi bond is formed between carbon 2 and 3 of the diene.

- One of the most important things to understand about this process is that it is concerted – all of the electron rearrangement takes place at once, with no carbocation intermediates.
- Since the diene takes the role of the nucleophile, electron donating group increase the reactivity of the diene. While the dienophile takes the role of the electrophile, electronwithdrawing groups increase the reactivity of the dienophile. The reactions below are examples of the Diels-Alder reaction.

Solved Examples
Example: Draw the bond-line structures for the reactions below.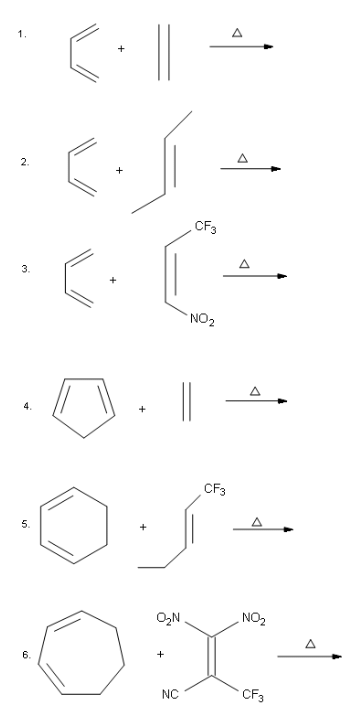 Ans:
Ans:

Diels-Alder Reactions are Stereospecific
- The Diels-Alder reaction is enormously useful for synthetic organic chemists, not only because ring-forming reactions are useful in general but also because in many cases two new stereocenters are formed, and the reaction is inherently stereospecific. A cis dienophile will generate a ring with cis substitution, while a trans dienophile will generate a ring with trans substitution:
 We noted earlier that addition reactions of alkenes often exhibited stereoselectivity, in that the reagent elements in some cases added syn and in other cases anti to the the plane of the double bond. Both reactants in the Diels-Alder reaction may demonstrate stereoisomerism, and when they do it is found that the relative configurations of the reactants are preserved in the product (the adduct). The following drawing illustrates this fact for the reaction of 1,3-butadiene with (E)-dicyanoethene. The trans relationship of the cyano groups in the dienophile is preserved in the six-membered ring of the adduct.
We noted earlier that addition reactions of alkenes often exhibited stereoselectivity, in that the reagent elements in some cases added syn and in other cases anti to the the plane of the double bond. Both reactants in the Diels-Alder reaction may demonstrate stereoisomerism, and when they do it is found that the relative configurations of the reactants are preserved in the product (the adduct). The following drawing illustrates this fact for the reaction of 1,3-butadiene with (E)-dicyanoethene. The trans relationship of the cyano groups in the dienophile is preserved in the six-membered ring of the adduct. - Likewise, if the terminal carbons of the diene bear substituents, their relative configuration will be retained in the adduct. Using the earlier terminology, we could say that bonding to both the diene and the dienophile is syn. An alternative description, however, refers to the planar nature of both reactants and terms the bonding in each case to be suprafacial (i.e. to or from the same face of each plane). This stereospecificity also confirms the synchronous nature of the 1,4-bonding that takes place.

Question for Diels-Alder Cycloaddition Rxn & its StereochemistryTry yourself: Which conformation is required for a Diels-Alder reaction to occur?View Solution
Diene Substituents and Diels-Alder Reactivity
- In order for a Diels-Alder reaction to occur, the diene molecule must adopt what is called the s-cis conformation:

- The s-cis conformation is higher in energy than the s-trans conformation, due to steric hindrance. For some dienes, extreme steric hindrance causes the s-cis conformation to be highly strained, and for this reason such dienes do not readily undergo Diels-Alder reactions.

Bicyclic Ring Formation and the Exo- and Endo- Positions
- Cyclic dienes that are ‘locked’ in the s-cis conformation are especially reactive. The result of a Diels-Alder reaction involving a cyclic diene is a bicyclic structure:

- Here, we see another element of stereopecificity: Diels-Alder reactions with cyclic dienes favor the formation of bicyclic structures in which substituents are in the endo position.

- The endo position on a bicyclic structure refers to the position that is inside the concave shape of the larger (six-membered) ring. As you might predict, the exo position refers to the outside position.
- The rate at which a Diels-Alder reaction takes place depends on electronic as well as steric factors. A particularly rapid Diels-Alder reaction takes place between cyclopentadiene and maleic anhydride.

- We already know that cyclopentadiene is a good diene because of its inherent s-cis conformation. Maleic anhydride is also a very good dienophile, because the electron-withdrawing effect of the carbonyl groups causes the two alkene carbons to be electron-poor, and thus a good target for reaction with the pi electrons in the diene.
- In general, Diels-Alder reactions proceed fastest with electron-donating groups on the diene (eg. alkyl groups) and electron-withdrawing groups on the dienophile.
- Alkynes can also serve as dienophiles in Diels-Alder reactions:

- Below are three examples of Diels-Alder reactions that have been reported in recent years:

Other Pericyclic Reactions
- The Diels-Alder reaction is just one example of a pericyclic reaction: this is a general term that refers to concerted rearrangements that proceed though cyclic transition states. Two well-studied intramolecular pericyclic reactions are known as the Cope rearrangement . . .
 . . .and the Claisen rearrangement (when an oxygen is involved):
. . .and the Claisen rearrangement (when an oxygen is involved):
- Notice that the both of these reactions require compounds in which two double bonds are separated by three single bonds.
- Pericyclic reactions are rare in biological chemistry, but here is one example: the Claisen rearrangement catalyzed by chorismate mutase in the aromatic amino acid biosynthetic pathway.

- The study of pericyclic reactions is an area of physical organic chemistry that blossomed in the mid-1960s, due mainly to the work of R.B. Woodward, Roald Hoffman, and Kenichi Fukui. The Woodward-Hoffman rules for pericyclic reactions (and a simplified version introduced by Fukui) use molecular orbital theory to explain why some pericyclic processes take place and others do not. A full discussion is beyond the scope of this text, but if you go on to study organic chemistry at the advanced undergraduate or graduate level you are sure to be introduced to this fascinating area of inquiry.
Diels-Alder Reaction Summary
The essential characteristics of the Diels-Alder cycloaddition reaction may be summarized as follows:
- The reaction always creates a new six-membered ring. When intramolecular, another ring may also be formed.
- The diene component must be able to assume a s-cis conformation.
- Electron withdrawing groups on the dienophile facilitate reaction.
- Electron donating groups on the diene facilitate reaction.
- Steric hindrance at the bonding sites may inhibit or prevent reaction.
- The reaction is stereospecific with respect to substituent configuration in both the dienophile and the diene.
These features are illustrated by the following eight examples, one of which does not give a Diels-Alder cycloaddition.
- There is no reaction in example D because this diene cannot adopt an s-cis orientation. In examples B, C, F, G & H at least one of the reactants is cyclic so that the product has more than one ring, but the newly formed ring is always six-membered. In example B the same cyclic compound acts as both the diene colored blue) and the dienophile (colored red).
- The adduct has three rings, two of which are the five-membered rings present in the reactant, and the third is the new six-membered ring (shaded light yellow). Example C has an alkyne as a dienophile (colored red), so the adduct retains a double bond at that location. This double bond could still serve as a dienophile, but in the present case the diene is sufficiently hindered to retard a second cycloaddition.
- The quinone dienophile in reaction F has two dienophilic double bonds. However, the double bond with two methyl substituents is less reactive than the unsubstituted dienophile due in part to the electron donating properties of the methyl groups and in part to steric hindrance. The stereospecificity of the Diels-Alder reaction is demonstrated by examples A, E & H. In A & H the stereogenic centers lie on the dienophile, whereas in E these centers are on the diene. In all cases the configuration of the reactant is preserved in the adduct.
Question for Diels-Alder Cycloaddition Rxn & its StereochemistryTry yourself: Which of the following statements is true about the Diels-Alder reaction?View Solution
The document Diels-Alder Cycloaddition Rxn & its Stereochemistry | Chemistry Optional Notes for UPSC is a part of the UPSC Course Chemistry Optional Notes for UPSC.
All you need of UPSC at this link: UPSC
FAQs on Diels-Alder Cycloaddition Rxn & its Stereochemistry - Chemistry Optional Notes for UPSC
| 1. What is the Diels-Alder (4+2) cycloaddition reaction? |  |
Ans. The Diels-Alder (4+2) cycloaddition reaction is a pericyclic reaction in organic chemistry where a diene reacts with a dienophile to form a cyclic compound. It involves the formation of two new sigma bonds and the breaking of one pi bond.
| 2. What is the mechanism of the Diels-Alder reaction? |  |
Ans. The mechanism of the Diels-Alder reaction involves concerted bonding changes between the diene and dienophile. The diene acts as a nucleophile attacking the electrophilic dienophile, resulting in the formation of a cyclic intermediate. This intermediate then undergoes a rearrangement to form the final product.
| 3. Can you provide some examples of the Diels-Alder reaction? |  |
Ans. Yes, here are some examples of the Diels-Alder reaction:
- The reaction between 1,3-butadiene and ethene to form cyclohexene.
- The reaction between 1,3-butadiene and acrylonitrile to form a substituted cyclohexene.
- The reaction between cyclopentadiene and maleic anhydride to form a bicyclic compound.
| 4. How does the stereochemistry of the Diels-Alder reaction work? |  |
Ans. The Diels-Alder reaction is stereospecific, meaning that the stereochemistry of the reactants determines the stereochemistry of the product. If the dienophile adds to the diene in a syn fashion, the product will have the same stereochemistry. If the addition occurs in an anti fashion, the product will have the opposite stereochemistry.
| 5. What are the other pericyclic reactions similar to the Diels-Alder reaction? |  |
Ans. Other pericyclic reactions similar to the Diels-Alder reaction include the [2+2] cycloaddition reaction and the [3+2] cycloaddition reaction. In the [2+2] cycloaddition, two double bonds react to form a four-membered ring, while in the [3+2] cycloaddition, three double bonds react to form a five-membered ring. These reactions also follow the Woodward-Hoffmann rules, which govern the stereochemistry and reactivity of pericyclic reactions.

|
Explore Courses for UPSC exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches




 We noted earlier that addition reactions of alkenes often exhibited stereoselectivity, in that the reagent elements in some cases added syn and in other cases anti to the the plane of the double bond. Both reactants in the Diels-Alder reaction may demonstrate stereoisomerism, and when they do it is found that the relative configurations of the reactants are preserved in the product (the adduct). The following drawing illustrates this fact for the reaction of 1,3-butadiene with (E)-dicyanoethene. The trans relationship of the cyano groups in the dienophile is preserved in the six-membered ring of the adduct.
We noted earlier that addition reactions of alkenes often exhibited stereoselectivity, in that the reagent elements in some cases added syn and in other cases anti to the the plane of the double bond. Both reactants in the Diels-Alder reaction may demonstrate stereoisomerism, and when they do it is found that the relative configurations of the reactants are preserved in the product (the adduct). The following drawing illustrates this fact for the reaction of 1,3-butadiene with (E)-dicyanoethene. The trans relationship of the cyano groups in the dienophile is preserved in the six-membered ring of the adduct. 







 . . .and the Claisen rearrangement (when an oxygen is involved):
. . .and the Claisen rearrangement (when an oxygen is involved):

















