The Making of Periodic Table | Chemistry Class 11 - NEET PDF Download
Why Do We Need To Classify Elements?
The Classification of Elements is essential because it allows us to organize our understanding of the properties and behaviors of various elements in a systematic way.
- In 1800, only 31 elements were known. By 1865, the number of identified elements had more than doubled to 63. At present 114 elements are known. Of them, the recently discovered elements are man-made.
- With the discovery of over 100 elements, it can be challenging to study each element and its compounds individually.
- By grouping elements based on shared characteristics such as atomic structure, electron configuration, and chemical reactivity, we can make predictions about their behavior and properties.
- This knowledge is crucial for fields such as materials science, medicine, environmental science, and engineering, as it allows us to develop new materials, treatments, and technologies.
- Ultimately, the classification of elements provides a foundation for our understanding of the physical world and helps us make sense of the complex interactions between matter and energy.
What is a Periodic Table?
The Periodic Table is a tabular representation of all the chemical elements arranged in order of their atomic number, electron configuration, and chemical properties.
- The elements are organized in rows and columns according to their atomic structure and properties, which allows for easy classification and understanding of their behavior.
- The table includes information on the elements' atomic number, symbol, name, and atomic mass, as well as other important data such as electron configuration, reactivity, and physical properties.
- The periodic table has become a fundamental tool for chemists and other scientists, helping them to predict the behavior of elements and their compounds and to design new materials with specific properties.
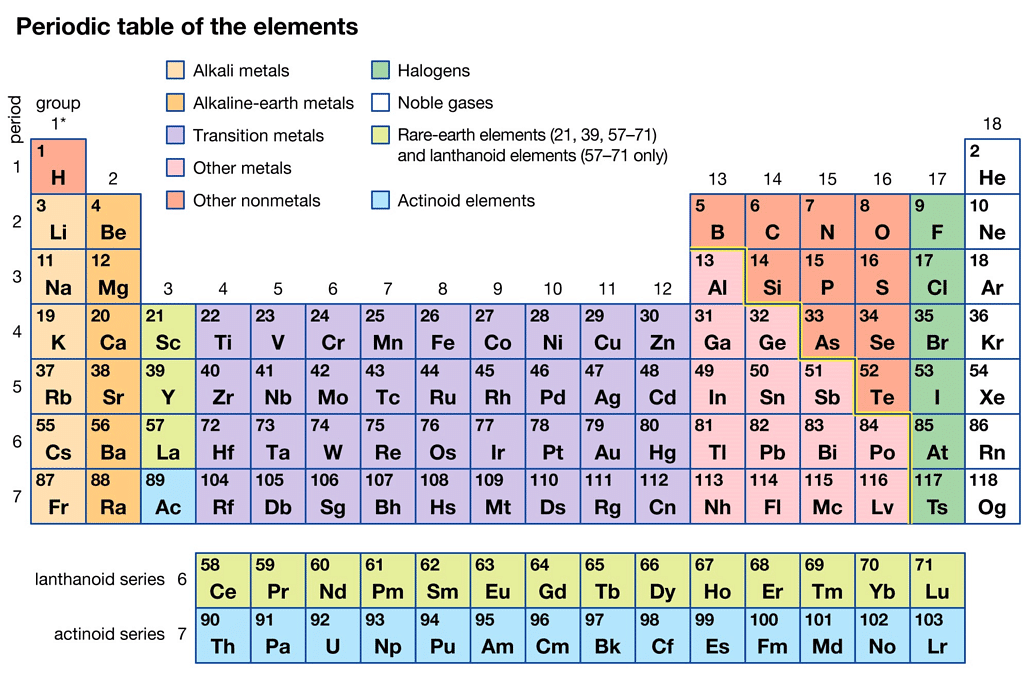 Modern Periodic Table
Modern Periodic Table
- The modern periodic table that we know today is the result of the work of many researchers who built on the ideas and theories of those who came before them.
Some of the most important theories or ideas in the development of the periodic table include Dobereiner's Triads, Newland's Law of Octaves, Lother Meyer's Classification, and Mendeleev's Periodic Table which made the modern periodic table.
In this Article, we will delve into each of these theories and ideas in detail.
1. Dobereiner's Triads
Dobereiner's Triads were an important early contribution to the development of the periodic table. He observed that certain elements had similar chemical properties and could be arranged into groups of three, or "triads."
Given below are the features of Dobereiner's Triads
- He arranged similar elements in the groups of three elements called triads, in which the atomic mass of the central element was merely the arithmetic mean of atomic masses of the other two elements or all the three elements possessed nearly the same atomic masses
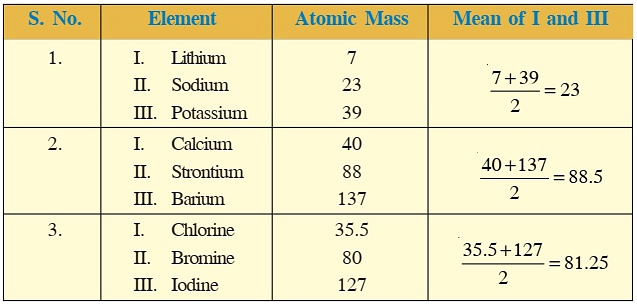 Dobereiner's Triads
Dobereiner's Triads
- For instance, sodium (Na), lithium (Li), and potassium (K) belong to a triad because the atomic weight of sodium is the sum of the atomic weights of lithium and potassium divided by two, which is (7+39)/2 = 23.
- These elements have similar properties such as reactivity with water and the ability to form salts with similar properties. Similarly, calcium (Ca), strontium (Sr), and barium (Ba) belong to another triad because they have similar chemical properties and atomic weights that follow the same pattern.
- Dobereiner arranged some elements into groups of three with similar properties, but he couldn't do it for all elements. There were some exceptions, like for very light or heavy elements. For example, the atomic mass of chlorine didn't fit into one of his groups. Despite this, his work was important for creating the periodic table.
Here are the merits and demerits of Dobereiner's Triads
Merits of Dobereiner's Triads
- Dobereiner's Triads were one of the first attempts to identify patterns in the properties of elements.
- For example, Dobereiner identified a triad consisting of chlorine, bromine, and iodine, which all had similar chemical properties and atomic weights.
By identifying patterns in the properties of elements, Dobereiner was able to make predictions about the properties of unknown elements, such as predicting the properties of the yet-undiscovered element, germanium.
Demerits of Dobereiner's Triads
- The main Limitation of Dobereiner's classification of elements was that he failed to arrange all the then-known elements in the form of triads of elements having similar chemical properties.
- Dobereiner could identify only three triads. He was not able to prepare triads of all the known elements
- For very low mass or for very high mass elements, the law was not holding good. Take the example of F, Cl, Br. Atomic mass of Cl is not an arithmetic mean of atomic masses of F and Br.
2. Newland's Law of Octaves
Newlands' Law of Octaves was a hypothesis proposed by John Alexander Reina Newlands in 1864 to classify the elements based on their atomic weights and chemical properties.
- Dobereiner's Triads and Newland's Law of Octaves are two early attempts to understand how elements are related to each other. Newland's Law of Octaves came after Dobereiner's Triads and built upon the idea of periodicity in the elements.
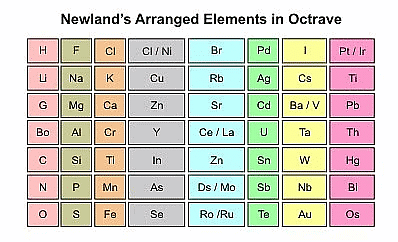
- Newland's arranged the known elements in order of their increasing atomic weights and grouped them into seven rows. He was the first to correlate the chemical properties of the elements with their atomic masses.
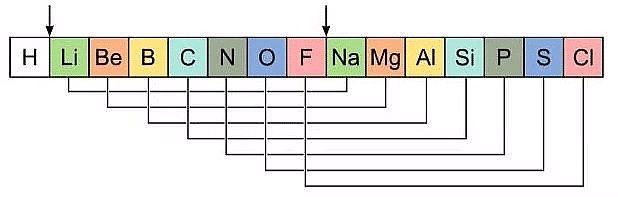 Newlands' Law of Octaves
Newlands' Law of Octaves
- He found that every eighth element in the list had similar chemical and physical properties to the first element, similar to the way that musical notes repeat every eighth note. Newlands called this the "Law of Octaves."
- For example, he found that lithium was similar in properties to sodium and potassium, which were the first and eighth elements in his list.
- Newlands' Law of Octaves was not widely accepted in the scientific community because it only applied to the known elements at the time and did not account for the discovery of new elements in the future.
- Additionally, Newlands' system was not consistent throughout the entire periodic table and did not account for the variation of properties within each row.
- Despite these criticisms, Newlands' Law of Octaves provided a foundation for future periodic table development and inspired the work of other scientists, such as Dmitri Mendeleev.
Here are the merits and demerits of Newlands' Law of Octaves
Merits of Newland's Law of Octaves
- Organized elements systematically based on their properties
- Laid the foundation for the development of the periodic table
- Simple and easy to understand
Demerits of Newland's Law of Octaves
- Limited range of applicability after calcium
- Inaccurate predictions and failed to predict the discovery of new elements
- Arbitrary classification based on observation of similarities, rather than scientific theory.
3. Lother Meyer's Classification
Lothar Meyer was a German chemist who independently developed a periodic table around the same time as Dmitri Mendeleev. Meyer's classification system was similar to Mendeleev's in that it arranged the elements in increasing atomic weight, with elements having similar chemical and physical properties placed in the same vertical column.
- He determined the atomic volumes by dividing atomic mass with its density in solid states.
- He plotted a graph between atomic masses against their respective atomic volumes for a number of elements.
- He found the following observations.
- Elements with similar properties occupied similar positions on the curve.
- Alkali metals having larger atomic volumes occupied the crests.
- Transitions elements occupied the troughs.
- The halogens occupied the ascending portions of the curve before the inert gases.
- Alkaline earth metals occupied the positions at about the midpoints of the descending portions of the curve.
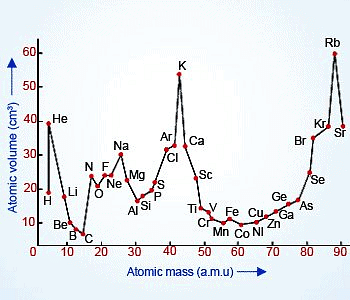
- On the basis of these observations, he concluded that the atomic volumes (a physical property) of the elements are a periodic function of their atomic masses. However, It was discarded as it lacks practical utility.
Here are the merits and demerits of Lother Meyer's Classification:
Merits of Lother Meyer's Classification:
- Based on atomic number, providing more accuracy
- Follows the periodic law and has high predictive power
- Simplifies the study of chemistry.
Demerits of Lother Meyer's Classification:
- Ignores isotopes and does not fully explain chemical bonding
- Was incomplete when first proposed and requires periodic updates
- Provides a general overview of the elements but lacks detailed information.
4. Mendeleev's Periodic Table
Dmitri Mendeleev was a Russian chemist who is credited with the creation of the first periodic table of the elements in 1869. His table organized the elements in order of increasing atomic mass and grouped elements with similar chemical properties together. Mendeleev's Periodic Table revolutionized the study of chemistry and laid the foundation for modern periodic tables that we use today.
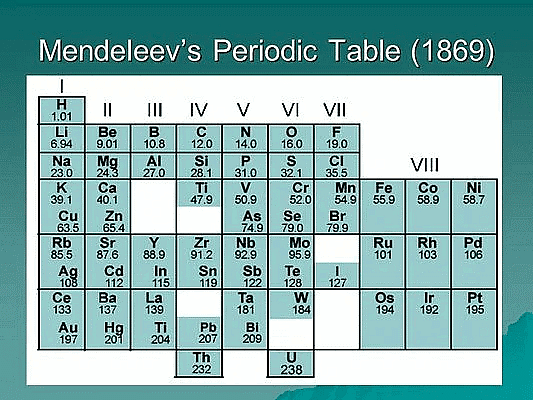
- Mendeleev's Periodic's Law: According to him the physical and chemical properties of the elements are a periodic function of their atomic masses. He arranged then known elements in order of their increasing atomic masses considering the fact that elements with similar properties should fall in the same vertical columns and leaving out blank spaces where necessary.
- Predictive power: Mendeleev's Periodic Table had a high predictive power, allowing Mendeleev to predict the existence and properties of undiscovered elements. For example, he predicted the existence of germanium and gallium, and his predictions were later confirmed by the discovery of these elements.
- Grouping of elements: Mendeleev's Periodic Table grouped elements with similar chemical properties together, providing a systematic organization of the elements. He identified several groups, including the alkali metals, alkaline earth metals, halogens, and noble gases, which are still used in modern periodic tables.
- Accommodation of new elements: Mendeleev's Periodic Table left gaps for elements that were yet to be discovered and provided a framework for accommodating new elements as they were discovered. This was possible because he recognized that the properties of elements were periodic functions of their atomic mass.
- Criticisms and limitations: Mendeleev's Periodic Table was not without criticisms and limitations. For example, the table did not account for the existence of isotopes, and the placement of some elements did not align with their chemical properties. However, these limitations were later addressed in the modern periodic tables that followed Mendeleev's.
Merits of Mendeleev's Periodic table:
1. It has simplified and systematized the study of elements and their compounds.
2. It has helped in predicting the discovery of new elements on the basis of the blank spaces given in its periodic table.
- Mendeleev predicted the properties of those missing elements from the known properties of the other elements in the same group.
- Eka - Aluminium, and Eka-silicon names were given for gallium and germanium (not discovered at the time of Mendeleev).
- Properties predicted by Mendeleev for these elements and those found experimentally were almost similar.
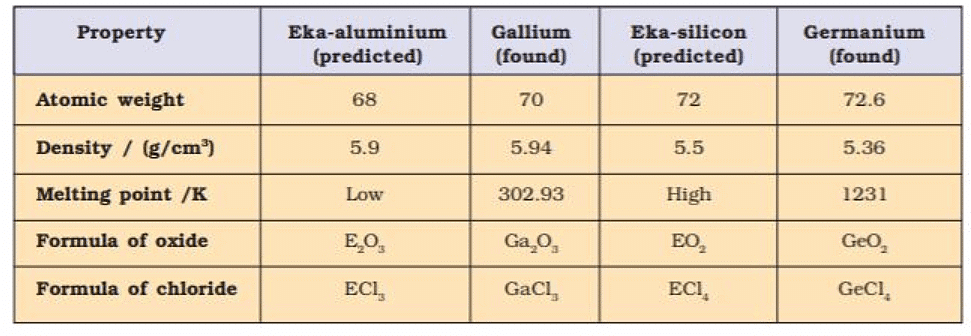
3. Atomic weights of elements were corrected. The atomic weight of Be was calculated to be 3 × 4.5 = 13.5 by considering its valency 3, was correctly calculated considering its valency 2 (2 × 4.5 = 9)
Demerits of Mendeleev's Periodic Table:
- Position of hydrogen is uncertain. It has been placed in group 1 and 17th group because of its resemblance with both groups.
- No separate positions were given to isotopes.
- Anomalous position of lanthanides and actinides in periodic table.
- Order of increasing atomic weights is not strictly followed in the arrangement of elements in the periodic table. For e.g.-Ar(At.wt.39.94) is placed before K(39.08) and Te (127.6) is placed before I (126.9)
- Similar elements were placed in different groups(CuIB and Hg IIB) and the elements with different properties were placed in the same groups(alkali metals IA and coinage metals IB)
- It didn't explain the cause of periodicity.
The Modern Periodic Table
The modern periodic table is a tabular arrangement of chemical elements based on their atomic number, electronic configuration, and chemical properties. It is an improvement of earlier periodic tables such as Mendeleev's and Meyer's tables. Here are some key points about the modern periodic table:
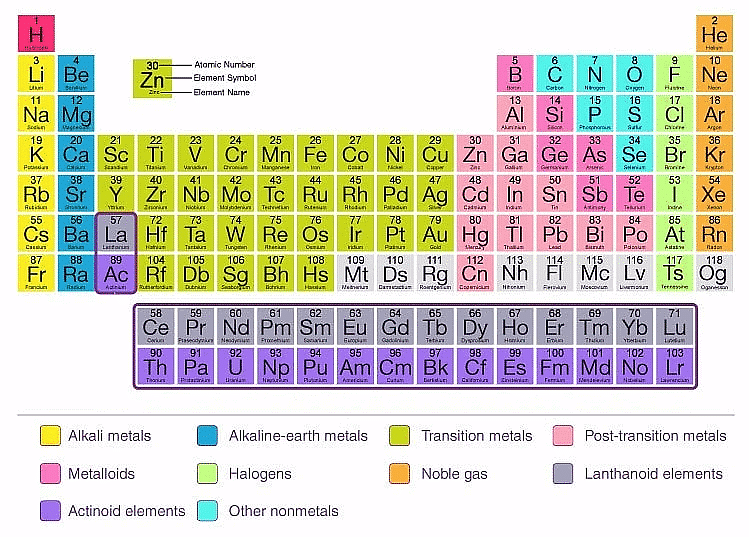
- Atomic number: The modern periodic table is arranged according to the increasing order of atomic number rather than atomic mass, as it provides a more accurate placement of elements with similar chemical properties.
- Periodic law: The modern periodic table follows the periodic law, which states that the chemical and physical properties of elements are a periodic function of their atomic number.
- Groups and periods: The modern periodic table is arranged in groups (vertical columns) and periods (horizontal rows). Elements in the same group share similar properties, while elements in the same period have the same number of electron shells.
- Classification of elements: The modern periodic table classifies elements into metals, non-metals, and metalloids based on their physical and chemical properties.
- Block classification: The modern periodic table also classifies elements into s, p, d, and f blocks based on the electronic configuration of their valence electrons. This helps in predicting the chemical and physical properties of elements.
- Usefulness: The modern periodic table is an essential tool for chemists, as it provides a systematic organization of the elements, predicts the properties of elements based on their position, and allows for the discovery and classification of new elements.
In conclusion, the modern periodic table is a fundamental tool in the study of chemistry and has been critical in the discovery and classification of new elements. Its systematic organization and predictive power make it an essential tool for researchers and students alike.
|
127 videos|244 docs|87 tests
|
FAQs on The Making of Periodic Table - Chemistry Class 11 - NEET
| 1. Why do we need to classify elements? |  |
| 2. What is a Periodic Table? |  |
| 3. What are Dobereiner's Triads? |  |
| 4. What is Mendeleev's Periodic Table? |  |
| 5. How does the Modern Periodic Table differ from Mendeleev's Periodic Table? |  |

|
Explore Courses for NEET exam
|

|


















