Electrochemical Cells, Galvanic Cells & Measurement of Electrode Potential | Chemistry for JEE Main & Advanced PDF Download
| Table of contents |

|
| Key Concepts |

|
| Electrochemical Cells |

|
| Galvanic Cell |

|
| Electrochemical cell and Gibbs Energy of the Reaction |

|
| Measurement of Electrode Potential |

|
Key Concepts
Electrochemical Cells
An electrochemical cell consists of two electrodes (metallic conductors) in contact with an electrolyte (an ionic conductor).
An electrode and its electrolyte comprise an Electrode Compartment.
Electrochemical Cells can be classified as:
(i) Electrolytic Cells: Cells in which a non-spontaneous reaction is driven by an external source of current.
(ii) Galvanic Cells: Cells which produce electricity as a result of a spontaneous cell reaction .
Note: In a galvanic cell, cathode is positive with respect to anode.
In a electrolytic cell, anode is made positive with respect to cathode.
Galvanic Cell
This cell converts chemical energy into electrical energy.
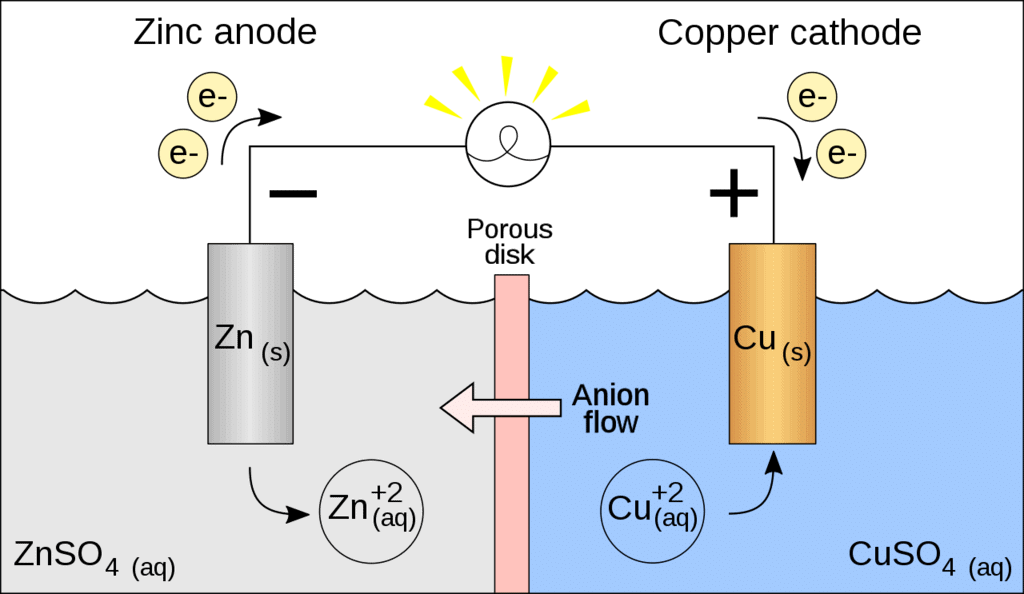 Galvanic Cell
Galvanic Cell
A galvanic cell is made up of two half cells i.e., anodic and cathodic. The cell reaction is of redox kind. Oxidation takes place at anode and reduction at cathode. It is also known as voltaic cell. It may be represented as shown in Fig. Zinc rod immersed in ZnSO4 behaves as anode and copper rod immersed in CuSO4 behaves as cathode.
Oxidation takes place at anode.
Zn → Zn2 + 2e- (loss of electron : oxidation)
Reduction takes place at cathode:
Cu2 + 2e- → Cu(gain of electron ; reduction)
Overall process: Zn(s) + Cu2+ → Cu(s) + Zn2+
In galvanic cell like Daniell cell: electrons flow from anode (zinc rod) to the cathode (copper rod) through external circuit; zinc dissolves as Zn2+ ; Cu2+ ion in the cathode cell picks up two electron and become deposited at cathode.
Representation of a cell (IUPAC Conventions):
Let us illustrate the convention taking the example of Daniel cell.
(i) Anodic half cell is written on left and cathodic half cell on right hand side.
Zn(s) |ZnSO4(sol)||CuSO4(sol)|Cu(s)
(ii) Two half cells are separated by double vertical lines: Double vertical lines indicate slat bridge or any type of porous partition.
(iii) EMF (electromotive force) may be written on the right hand side of the cell.
(iv) Single vertical lines indicate the phase separation between electrode and electrolyte solution.
Zn|Zn2+ ||Cu2+ |Cu
(v) Invert eletrodes are represented in the bracket
Zn|ZnSO4||H |H2,Pt
Electrochemical cell and Gibbs Energy of the Reaction
Electrical work done in one second is equal to electrical potential multiplied by total charge passed. If we want to obtain maximum work from a galvanic cell then charge has to be passed reversibly. The reversible work done by a galvanic cell is equal to decrease in its Gibbs energy and therefore if the emf of the cell is E and nF is the amount of charge passed and ΔrG is the Gibbs energy of the reaction, then
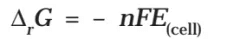 It may be remembered that E(cell) is an intensive parameter but ΔrG is an extensive thermodynamic property and the value depends on n. Thus, if we write the reaction
It may be remembered that E(cell) is an intensive parameter but ΔrG is an extensive thermodynamic property and the value depends on n. Thus, if we write the reaction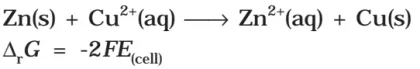 But when we write the reaction
But when we write the reaction
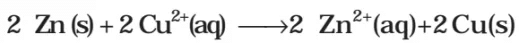
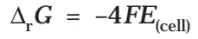
If the concentration of all the reacting species is unity, then E(cell)
Thus, from the measurement of E–Cell we can obtain an important thermodynamic quantity, ΔrG–, standard Gibbs energy of the reaction.From the latter we can calculate equilibrium constant by the equation: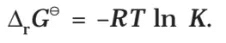
Relationship between ΔG and electrode potential:
Let n, Faraday charge is taken out from a cell of e.m.f. (E) then electrical work done by the cell may be calculated as,
Work done = Charge × Potential = nFE
From thermodynamics we know that decrease in Gibbs free energy of a system is a measure of reversible or maximum obtainable work by the system if there is no work due to volume expansion
ΔG = - nFE
Under standard state ΔGº = - nFEº ............(1)
(i) From thermodynamics we know, DG = negative for spontaneous process. Thus from eq. (i) it is clear that the EMF should be ve for a cell process to be feasible or spontaneous.
(ii) When ΔG = positive, E = negative and the cell process will be non spontaneous.
Reactions | ΔG | E |
Spontaneous | (-) | (+) |
Non-spontaneous | (+) | (-) |
Equilibrium | 0 | 0 |
Standard free energy h, range of a cell may be calculated by electrode potential data.
Substituting the value of Eº (i.e., standard reduction potential of cathode-standard reduction potential of anode) in eq. (i) we may get ΔGº.
Measurement of Electrode Potential
Concept of electromotive force (EMF) of A Cell
Electron flows from anode to cathode in external circuit due to a pushing effect called or electromotive force (e.m.f.). EMF is called as cell potential. Unit of e.m.f. of cell is volt.
EMF of cell may be calculated as:
Ecell = reduction potential of cathode - Reduction potential of anode
Similarly, standard e.m.f. of the cell (Eº) may be calculated as
Eºcell = Standard reduction potential of cathode - Standard reduction potential of anode.
Sign Convention of EMF
EMF of cell should be positive other wise it will not be feasible in the given direction.
Zn|ZnSO4||CuSO4|Cu , E = 1.10 volt (Feasible)
Cu|CuSO4||ZnSO4|Zn, E = - 1.10 volt (Not Feasible)
Salt Bridge
Two electrolyte solutions in galvanic cells are separated using salt bridge as represented in the Fig. Salt bridge is a device to minimize or eliminate the liquid junction potential. Saturated solution of salt like KCl, KNO3, NH4Cl and NH4NO3 etc. in agar-agar gel is used in salt bridge.
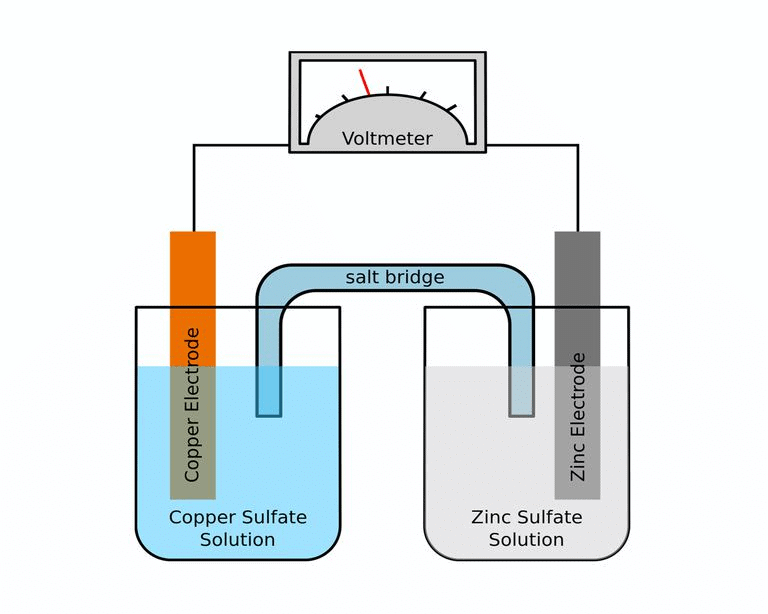 Fig: Salt bridgeSalt bridge contains high concentration of ions viz. K+ and NO3- at the junction with electrolyte solution. Thus, salt bridge carries whole of the current across the boundary; more over the K+ and NO3- ions have same speed. Hence, salt bridge with uniform and same mobility of cations and anions completes the electrical circuit & permits the ions to migrate.
Fig: Salt bridgeSalt bridge contains high concentration of ions viz. K+ and NO3- at the junction with electrolyte solution. Thus, salt bridge carries whole of the current across the boundary; more over the K+ and NO3- ions have same speed. Hence, salt bridge with uniform and same mobility of cations and anions completes the electrical circuit & permits the ions to migrate.
|
366 videos|853 docs|301 tests
|
FAQs on Electrochemical Cells, Galvanic Cells & Measurement of Electrode Potential - Chemistry for JEE Main & Advanced
| 1. What is an electrochemical cell and how does it work? |  |
| 2. What is a galvanic cell and how is it different from an electrolytic cell? |  |
| 3. How is the Gibbs energy of a reaction related to an electrochemical cell? |  |
| 4. How is the electrode potential of a cell measured? |  |
| 5. What are some practical applications of electrochemical cells? |  |





















