Electric Charges: Definition, Formula, Properties, Unit & More | Physics Class 12 - NEET PDF Download
| Table of contents |

|
| What is an Electric Charge? |

|
| Properties of Electric Charge |

|
| Conductors and Insulators |

|
| Concept-Based Questions |

|
| Frequently Asked Questions |

|
All of us have the experience of seeing a spark or hearing a crackle when we take off our synthetic clothes or sweaters, particularly in dry weather. Have you ever tried to find any explanation for this phenomenon? It can be attributed to electric charges.
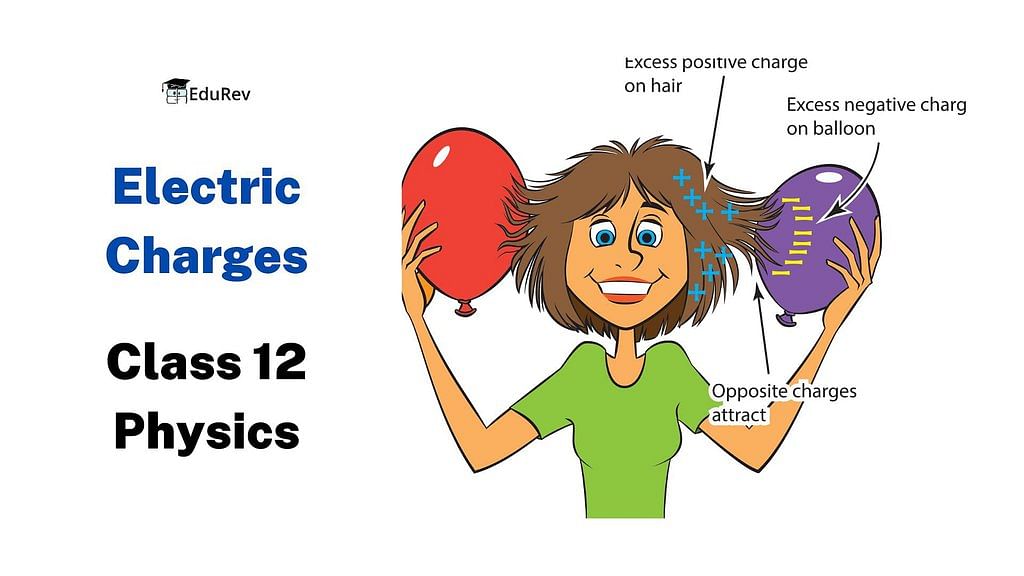
In this EduRev document, we will delve into the concepts of Electric Charges and Fields, specifically focusing on their definition, formula, properties, unit, and more, as covered in Class 12 Physics.
What is an Electric Charge?
Electric charge is a fundamental property of matter that determines how it interacts with other charged matter and electric fields. This property is carried by subatomic particles, with protons carrying a positive charge, electrons a negative charge, and neutral particles having no charge.
- The word "electricity" comes from the Greek word "elektron," which means amber. This word refers to the ancient observation of static electric charges generated by rubbing amber.
Types of Electric Charges
Two kinds of electric charges are there:
- Positive(+) charge
- Negative(-) charge
Positive Charge: When an object has a positive charge it means that it has more protons than electrons.
Negative Charge: When an object has a negative charge it means that it has more electrons than protons.
When there is an identical number of positive and negative charges, the negative and positive charges would cancel out each other and the object would become neutral.

How to Measure an Electric Charge?
The electric charge is measured using a coulomb.
“One coulomb is the quantity of charge transferred in one second.”
Mathematically, the definition of a coulomb is represented as:
Q = I.t
In the equation, Q is the electric charge, I is the electric current and t is the time.
Unit of Electric Charge
A charge is a derived physical quantity. The charge is measured in coulomb in the S.I. unit.
In practice we use:
- millicoulomb mC (10-3 C)
- microcoulomb μC (10-6 C)
- nanocoulombs nC (10-9 C)
- C.G.S unit of charge = electrostatic unit = esu
- 1 coulomb = 3 × 109 esu of charge
- Dimensional formula of charge = [M0L0T1A1]
Note:
- Charge of a single electron = -1.602 × 10-19 C
- Charge of a single proton = + 1.602 × 10-19 C
- Charge of a single neutron = 0 C
Is Electric Charge a Vector Quantity?
No, electric charge is a scalar quantity.
- Apart from having a 'magnitude' and 'direction', for a quantity to be termed a vector (which we will study in detail later) it should also obey the laws of vector addition such as triangle law of vector addition and parallelogram law of vector addition, only then the quantity is said to be a vector quantity.
- Imagine you have two charges:
Charge C (4 coulombs of positive charge)
Charge C (2 coulombs of negative charge) - When we combine these two charges, we can simply add them algebraically:
Qtotal=Q1+Q2
Substituting the values:
Qtotal=+4C + (−2C) = +4C−2C=+2C
The total charge
Notice that we only considered the numerical values and their signs (positive or negative) when adding. There was no need to consider any direction associated with the charges. This illustrates that charge is a scalar quantity: it only has magnitude (how much charge) and sign (positive or negative), but no direction.
Properties of Electric Charge
1. Like charges repel each other and unlike charges attract each other. (As Electric Charge comes in two varieties, which are called “plus” and “minus”.)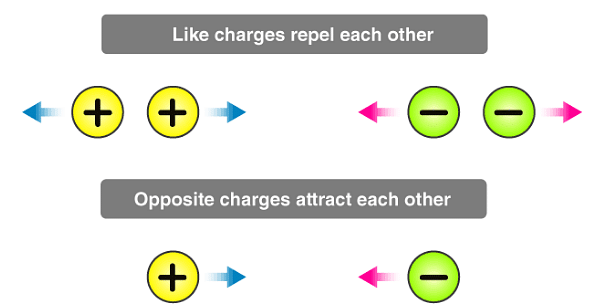
2. Electric Charge is a scalar quantity: It follows scalar laws of operations, i.e. it adds algebraically and represents the excess of electrons in a negatively charged atom or a deficiency of electrons in a positively charged atom.
3. A charge is transferable: Electric charge can be transferred from one body to another, but there is a restriction to the charge transfer. Only electrons are transferred from one body to another because protons are tightly bound to the nucleus of every atom. Hence, the body which loses electrons in the transfer becomes positively charged, and the body which receives electrons becomes negatively charged.
- A neutral body has a number of electrons = number of protons
- A positively charged body has a number of electrons < number of protons
- A negatively charged body has a number of electrons > number of protons.
4. Charge is always conserved: In an isolated system, the total charge (sum of positive and negative) remains constant whatever charge transfer takes place in the system internally. It is called the principle of charge conservation.
- Recently, the existence of particles of charge +(2/3) e and -(1/3) e has been postulated. These particles are called quarks, but still, this is not considered as the quantum of charge because these are unstable (They have a very short span of life.)
6. Charge is always associated with mass: Yes! Electrons, Protons and Neutrons also have masses.
Their value is determined, experimentally, to be following:
Mass of an electron = 9.109 × 10-31 Kg = 5.49 × 10-4 amu
Mass of a proton = 1.6726 × 10-27 Kg = 1.007 amu
Mass of a neutron = 1.6749 × 10-27 Kg = 1.008 amu
- It is recommended to remember these values in Kg (SI units). Also, please note that the mass of a neutron is slightly greater than the mass of a proton.
- This also shows that the mass of a negatively charged body is greater than the mass of a positively charged identical body as it would have an excess number of electrons than the positively charged bodies.
7. Charge is relativistically invariant: This means that charge is independent of the frame of reference, i.e., the charge on a body does not change whatever be its speed. This property is worth mentioning as in contrast to charge, the mass of a body depends on its speed and increases with an increase in speed. You will be exposed to this property later when you will learn The Special Theory of Relativity.
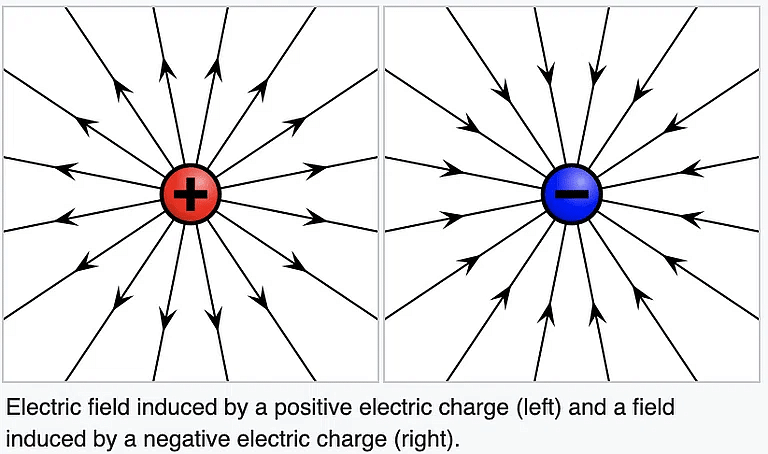
8. A charge at rest produces an only an electric field around itself: A charge at rest creates a region of influence called an Electric Field around itself in space.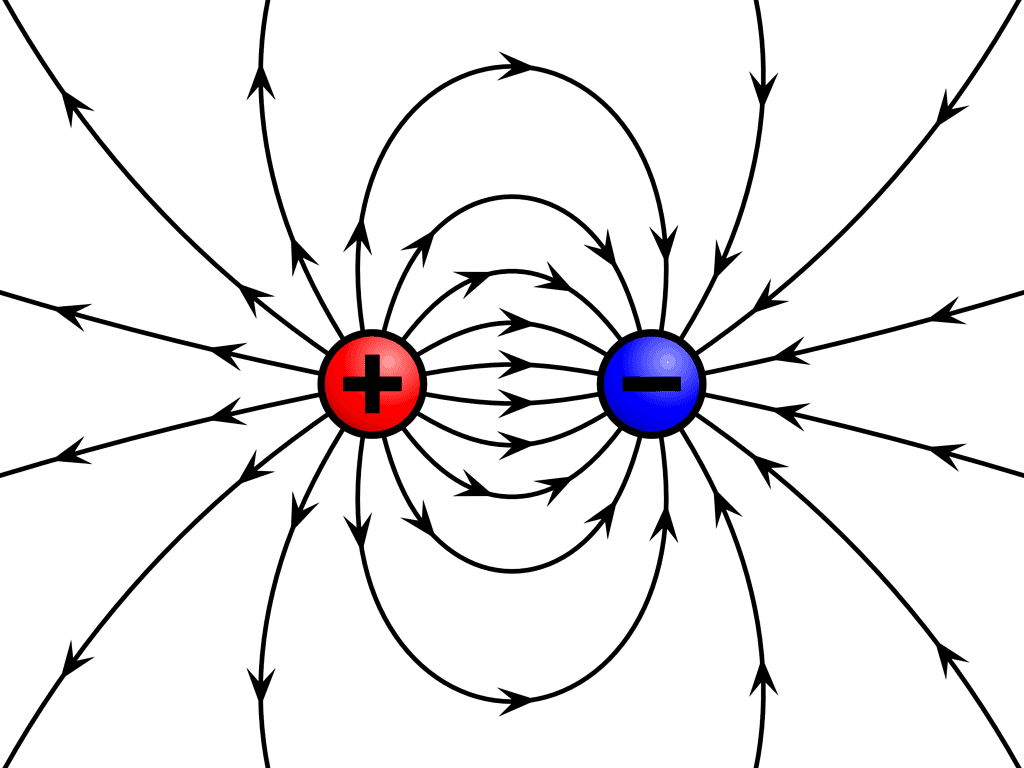
- While a charge having uniform motion (constant velocity) produces electric as well as the magnetic field around itself.
- Accelerated charges produce a special combination of electric and magnetic fields called electromagnetic waves.
- We will study the electric field in detail in the coming section.
Some Activities to Understand Charge
- Balloon attraction: Blow up a balloon and rub it against your hair or a woolen sweater. Then hold it near small bits of paper or Styrofoam. You will see that the balloon attracts these small objects, demonstrating the presence of static electricity.
 Ballon attraction
Ballon attraction - Salt and pepper attraction: Sprinkle salt and pepper on a table and then rub a plastic comb through your hair. Hold the comb near the salt and pepper and observe what happens. You will see that the salt and pepper move towards the comb, demonstrating the attraction of opposite charges.
 Salt and pepper attraction
Salt and pepper attraction - Lemon battery: Cut a lemon in half and remove the pulp, leaving only the skin intact. Insert a copper wire into one half of the lemon and a zinc wire into the other half. Then connect the wires using a small LED light. The lemon will generate enough electricity to light up the LED, demonstrating the production of electric charges.
 Lemon battery
Lemon battery - Comb and small pieces of paper: Rub a plastic comb against a woolen cloth or fur, and then hold the comb close to small pieces of paper or confetti. Observe as the pieces of paper are attracted to the comb and stick to it, demonstrating the presence of static electricity.

Static Electricity
Static electricity refers to an imbalance between the electric charges in a body, specifically the imbalance between the negative and the positive charges on a body.
- The imbalance in the charge is introduced by physical means. One of the most common causes of static electricity is contact between solid objects. It was mentioned earlier that the movement of protons is not possible and the only movement of electric charge seen in static electricity is electrons.
- Electrons in materials are held extremely loosely meaning that they can be exchanged through simple contact like rubbing.
- The image below is an example of rubbing a glass rod with silk which causes static electricity. When two objects are rubbed together to create static electricity, one object gives up electrons and becomes more positively charged while the other material collects electrons and becomes more negatively charged.
- We should keep in mind that the rules such as like charges repel and unlike charges attract is applicable here.
Conductors and Insulators
Any object can be broadly classified in either of the following two categories on the basis of their electrical properties:
(i) Conductors
(ii) Insulators
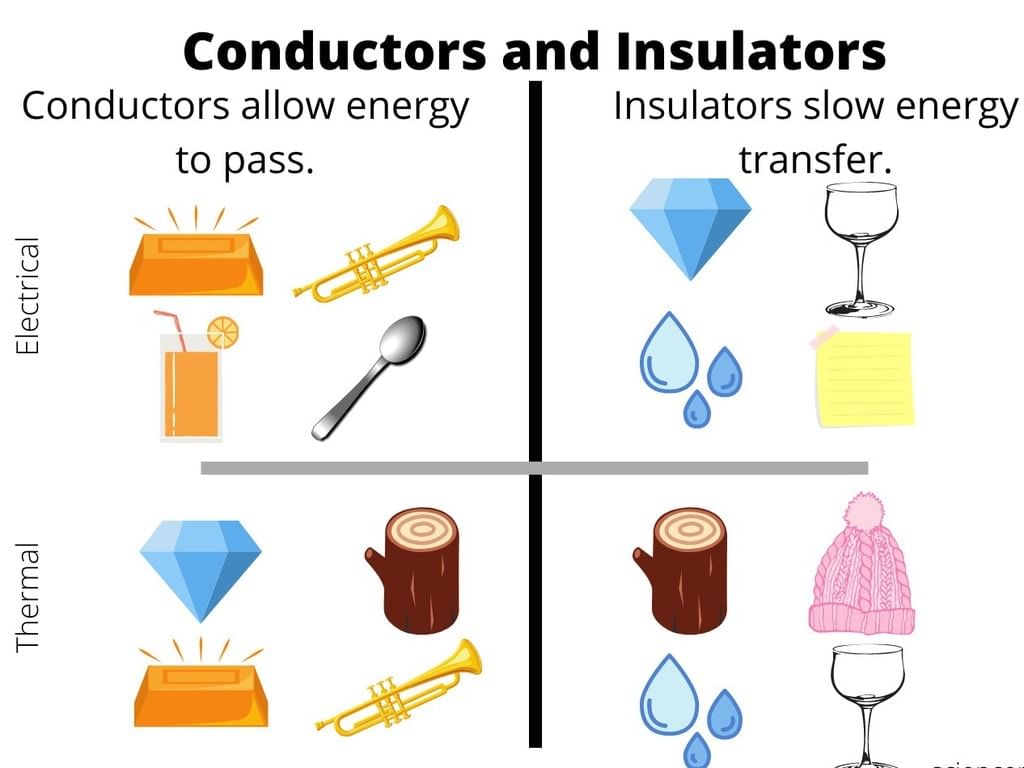
(i) Conductors: The materials or substances which allow electricity to flow through them are called Conductors. Conductors are able to conduct electricity because they allow electrons to flow inside them very easily.
The general property of conductor is to allow the transition of heat or light from one source to another. Metals, humans, earth and animal bodies fall in the category of conductors. This category generally comprises of metals but may sometimes contain non-metals too.
Example: Carbon in the form of graphite. Conductors have free electrons on its surface which allows current to pass through, that’s why conductors are able to conduct electricity.
Applications of Conductors
Conductors are quite useful in many ways and used in many real life applications like:
- Mercury is used in thermometer to check temperature of body.
- Aluminium is used in making foils to store food and also in production of fry pans to store heat quickly.
- Iron is used in vehicle engine to conduct heat.
- The plate of an iron is made up of steel to absorb heat briskly.
- Conductors are used in car radiators to eradicate heat away from the engine.

(ii) Insulators: The materials or substances which resist or don’t allow the current to flow through them are called Insulators. Insulators are mostly solid in nature and are used in a variety of systems. Insulators don’t allow the flow of heat as well.
The property which makes insulators different from conductors is its resistivity. Wood, cloth, glass, mica, and quartz are some good examples of insulators. Insulators are also called Protectors as they give protection against heat, sound and of course passage of electricity.
Insulators don’t have any electrons in its and that’s why insulators don’t conduct electricity.
Examples:
- Glass is the best insulator as it has the highest resistivity.
- Plastic is a good insulator and is used in making number of things.
- Rubber which is used to make tyres, fire-resistant clothes and slipper is a very good insulator.
Applications of Insulators

Being resistive to flow of electron, insulators are used worldwide in a number of ways.
Some are as follows:
- Thermal Insulators, disallow heat to move from one place to another and is used in making thermoplastic bottles, in fireproofing ceilings and walls.
- Sound Insulators help in controlling noise level, as they are good in absorbance of sound and are used in buildings, conference halls, and buildings to make them noise free.
- Electrical Insulators, which hinders flow of electron or passage of current through them are extensively used in circuit boards, high-voltage systems and also in coating electric wire and cables.
Concept-Based Questions
Q.1. Why have we defined only two types of charges? Why not three or more?
Ans. Only two kinds of electric charges exist because any unknown charge that is found experimentally to be attracted to a positive charge is also repelled by a negative charge. No one has ever observed a charged object that is repelled by both a positive and a negative charge.
Q.2. What happens
(a) When two like charges are brought together?
(b) When two, unlike charges, are brought together?
Ans. (a) When two like charges are brought together, they repel each other with an electrostatic force.
(b) When two, unlike charges, are brought together, they attract each other with an electrostatic force.
Q.3. What does neutral in electric charge mean?
Ans. Neutral does not refer to any third type of charge. It is the absence of any excess or deficiency of electrons in a body, i.e. the number of electrons = number of protons.
Q.4. Does the mass of the body get affected while charging?
Ans. Yes, the mass of the body gets affected because electrons have a definite mass, so the mass of the body slightly increases when it gains electrons while the mass decreases when it loses electrons.
Q.5. Two identical metallic spheres of exactly equal masses are taken. One is given a positive charge q coulombs and the other an equal negative charge. Are their masses after charging equal?
Ans. No, A body is positively charged due to the deficit of electrons while the negative charge is due to a surplus of electrons. Hence, the mass of the negatively charged sphere will be slightly more than that of the positively charged sphere.
Q.6. During a nuclear reaction, what happens to electric charge?
Ans. In the event of a nuclear reaction, the electric charge is conserved considering an isolated system. This is true for any nuclear or chemical reaction. In a nuclear reaction, the parent nuclei undergo a transformation into daughter nuclei, but the total algebraic charge remains constant.
Q.7. Explain the statement: ‘For a body, an electric charge is quantized.
Ans. Considering a particular body, ‘electric charge is quantized’ refers to the number of electrons which can be transferred from that body to another. It should be noted that charges don’t get transported in fractions. Therefore, the overall charge on a body is simply an integral multiple of charge on an electron.
Frequently Asked Questions
- How are electric charges distributed within the atom?
Ans. Subatomic particles carry electric charges. Electrons carry the negative charge and protons carry the positive charge in the nuclei of atoms. - Why is an electric charge a scalar quantity?
Ans. When two currents meet at a junction, the resultant current of these will be an algebraic sum and not the vector sum. Therefore, an electric current is a scalar quantity. - When will an electric charge be negative?
Ans. When the matter has more electrons than protons then it is said to have a negative charge. - When will an electric charge be positive?
Ans. When the matter has more number of protons than electrons, then it is said to have a positive charge. - What is the unit for measuring electric charge?
Ans. Coulomb is the unit for measuring electric charge.
|
97 videos|369 docs|104 tests
|
FAQs on Electric Charges: Definition, Formula, Properties, Unit & More - Physics Class 12 - NEET
| 1. What is the definition of electric charge? |  |
| 2. What are the types of electric charges? |  |
| 3. What is the unit of electric charge? |  |
| 4. Is electric charge a vector quantity? |  |
| 5. What is the difference between conductors and insulators? |  |

|
Explore Courses for NEET exam
|

|





















