Dual Nature Of Radiation And Matter | Physics Class 12 - NEET PDF Download
What is Electron Emission?
- When light is incident on a metal surface, it was observed that electrons are ejected from a metal surface sometimes even when incredibly dim light such as that from stars and distant galaxies, is incident on it, and sometimes electrons do not come out from the metal surface even high energetic or high-intensity light falling on the metal surface.
- This shows that the electron emission from a metal surface does not depend on the intensity of incident light but it basically depends on the energy of the incident.
- Even if the number of photons is very low in dim light, the photoelectric effect can be seen.
- During the photoelectric effect phenomenon, each incident photon on the metal surface can eject at most one electron.
- A photon is an energy packet that is fully absorbed, not partially. Thus, one photon can not be absorbed by more than one electron.
- The minimum amount of energy of photon required to eject an electron out of a metal surface is called work function. It is denoted by φ.
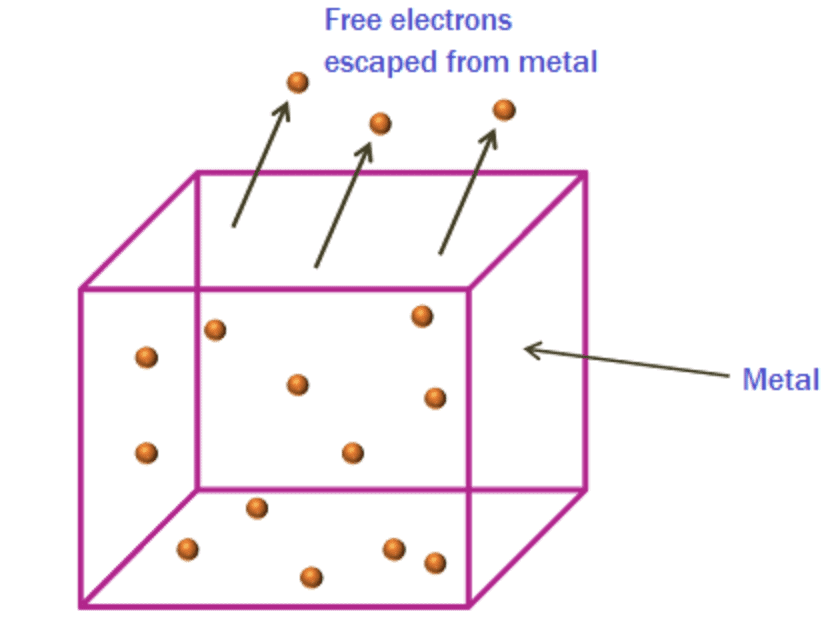
However, the surface barrier can be broken by providing a certain minimum amount of energy to the free electrons which increases their kinetic energy and consequently help them escape the metal surface.
Types of Electron Emission
The electron emission is possible only if sufficient energy (equal to the work function of the metal) is supplied to the metal in the form of heat energy, light energy, etc. Depending on the source of energy, electron emission can be of the following types:
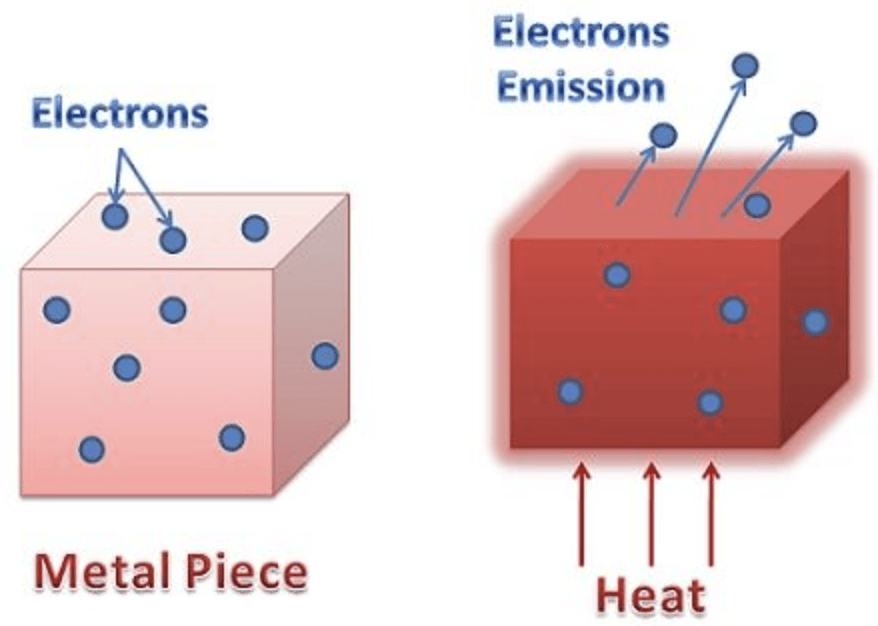
1. Thermionic Emission
In this type, the metal is heated to a sufficient temperature to enable the free electrons to come out of its surface.
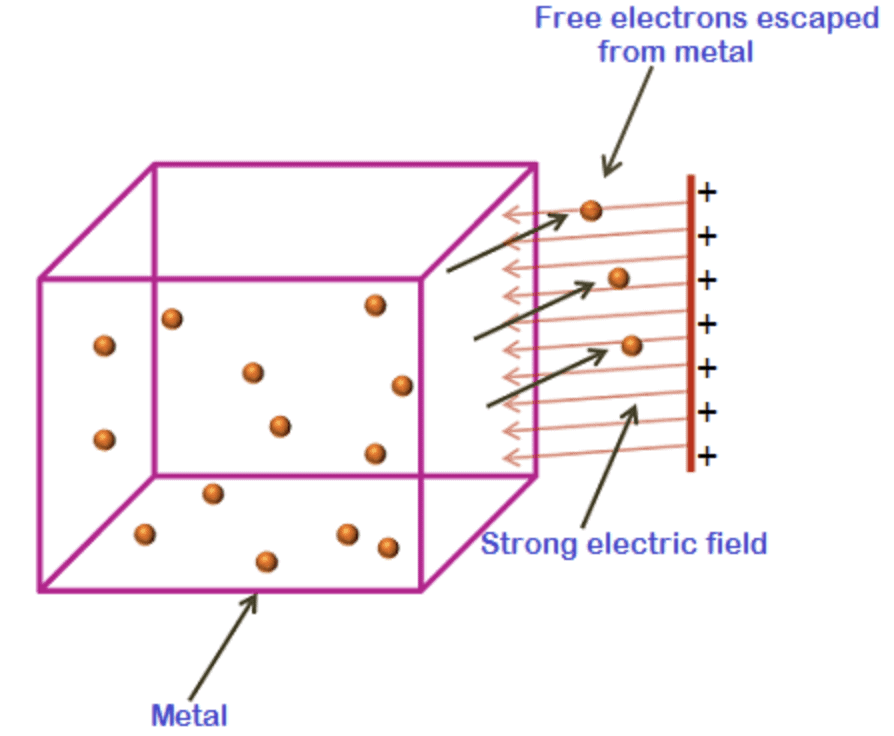
2. Field Emission
In field emission, a strong electric field is applied to the metal surface. This intense electric field exerts a force on the electrons, pulling them out of the metal. The positive charges in the metal lattice attract the electrons, facilitating their emission.
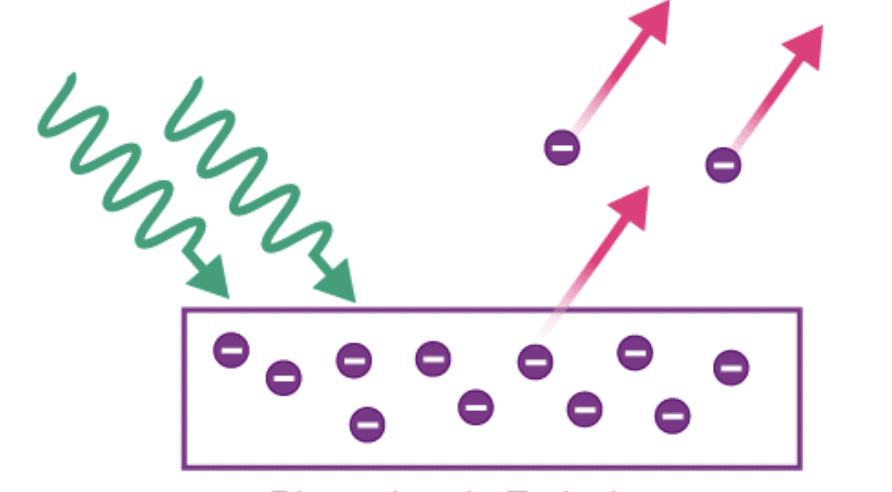
3. Photoelectric Emission
Photoelectric emission occurs when light of a specific frequency (or higher) strikes the surface of the metal. The energy from the photons in the light can be sufficient to overcome the work function, causing electrons to be emitted from the metal surface. This phenomenon is dependent on the frequency of the incident light, not just its intensity.
Photoelectric Effect
The photoelectric effect refers to the phenomenon where electrons are emitted from the surface of a metal when it is exposed to light or other forms of electromagnetic radiation with sufficient frequency, typically in the ultraviolet or visible light range.
- The emitted electrons are called photoelectrons, and the resulting current produced by these electrons is known as photoelectric current.
- Certain alkali metals, such as lithium and sodium, can exhibit the photoelectric effect when exposed to visible light. In contrast, other metals like zinc and cadmium require ultraviolet light to trigger the effect.
Note: Non-metals, liquids, and gases can also undergo the photoelectric effect, but this occurs under specific conditions and is generally less efficient than in metals. However, the extent of the photoelectric effect in non-metals can vary significantly compared to metals.
Photoelectric Effect Experiment
- The experimental setup for investigating the photoelectric effect involves a sealed glass or quartz tube. Inside this tube, there are two key components: a photosensitive plate known as the emitter and a metal plate called the collector.
- A transparent quartz window is fitted onto the tube, permitting ultraviolet light to enter and strike the photosensitive plate (C).
- When the photosensitive plate (C) is exposed to ultraviolet radiation, it releases electrons. These emitted electrons are then collected by the metal plate (A).
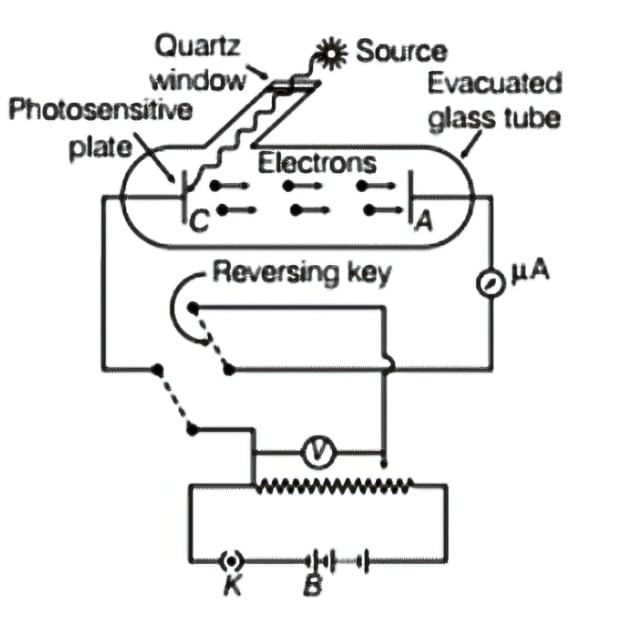 Experimental arrangement for the study of photoelectric effect
Experimental arrangement for the study of photoelectric effect - If the collector plate (A) is positively charged in relation to the emitter plate (C), it attracts the emitted electrons. This movement of electrons generates an electric current known as the photoelectric current within the circuit.
- The potential difference between the emitter and collector plates is measured using a voltmeter (V), while the resulting photocurrent in the circuit is monitored with a microammeter (μA).
- This experimental configuration enables the examination of how the photocurrent varies with different intensities and frequencies of incoming radiation. It also allows for the investigation of the effects of the potential difference between plates A and C on the photoelectric current.
Effect of Intensity of Light on Photoelectric Current
- When both the frequency of the incoming light and the accelerating potential are kept constant, the photoelectric current increases linearly with the intensity of the light.
- This indicates that the photoelectric current is directly proportional to the number of photoelectrons emitted per second, which depends on the intensity of the light.
Effect of Potential on Photoelectric Current
- When the frequency and intensity of the incoming light are held constant, the photoelectric current strengthens as the potential applied to the collector becomes more positive.
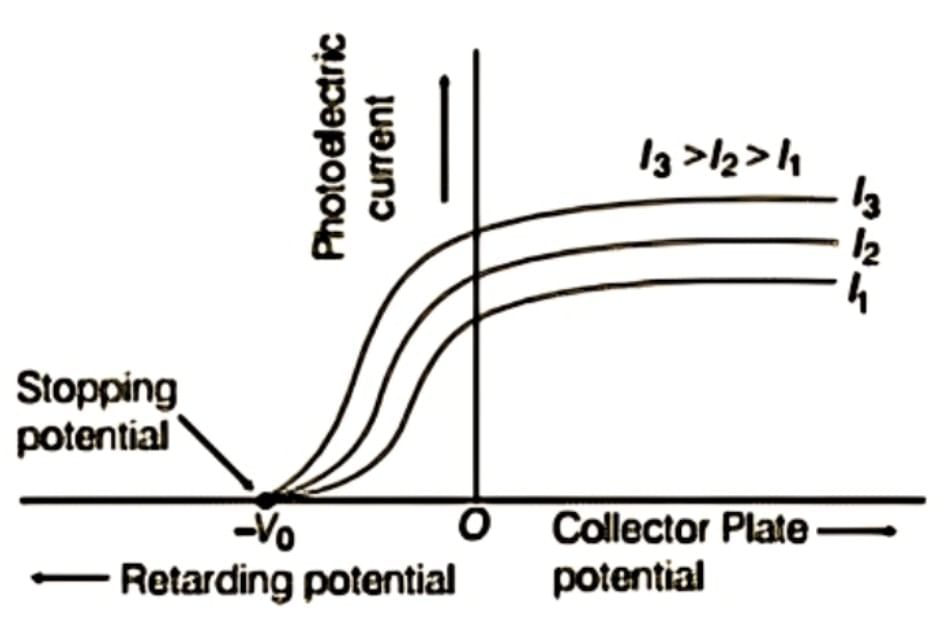 Variation of photoelectric current versus potential for different intensities but constant frequency
Variation of photoelectric current versus potential for different intensities but constant frequency
From the graph, we can observe:
- Saturation Current: When the accelerating potential reaches a certain threshold, all emitted photoelectrons can reach the collector plate, resulting in a stable photocurrent known as the saturation current.
- Current without Accelerating Potential: When the potential is reduced to zero, the current decreases but does not drop to zero. This indicates that some photoelectrons still reach the collector plate because they possess enough energy to overcome the material's work function.
- Stopping Potential (Cut-Off Potential): For a specific frequency of incoming light, applying a minimum negative potential (V0) to the collector plate causes the photoelectric current to cease. This minimum negative potential, where even the most energetic photoelectrons cannot reach the collector, is known as the stopping potential or cut-off potential.
- The stopping potential is directly related to the maximum kinetic energy (Kmax) of the photoelectrons, as illustrated in the equation:
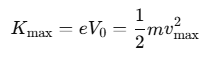
- In this equation, m represents the mass of the photoelectron, and vmax denotes the maximum velocity of the emitted photoelectrons.
Effect of Frequency of Incident Radiation on Stopping Potential
When radiation of different frequencies (but the same intensity) is used, the photoelectric current varies with the potential difference between the plates, as shown in the graph.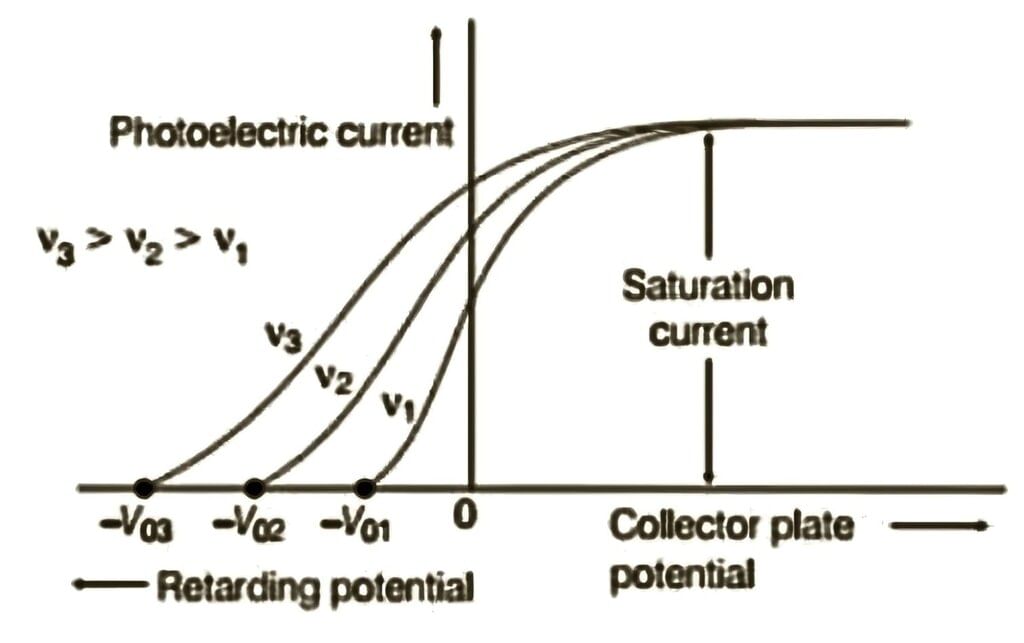 Variation of photoelectric current versus potential for different frequencies but constant intensity of incident radiation
Variation of photoelectric current versus potential for different frequencies but constant intensity of incident radiation
From the graph, we observe:
1. Stopping Potential and Frequency: The stopping potential varies with the frequency of the radiation but the saturation current remains constant for a given intensity.
2. Higher Frequency and Higher Stopping Potential: The stopping potential is more negative for higher-frequency radiation, indicating that the kinetic energy of emitted electrons depends on the radiation frequency. Higher frequencies result in higher kinetic energies for the photoelectrons, requiring a greater stopping potential to halt them.
3. Saturation Current and Intensity: The saturation current depends on the intensity of the radiation but is independent of the frequency.
If we plot stopping potential against the frequency of incident radiation for two different metals, we get a linear graph for each metal. This graph shows: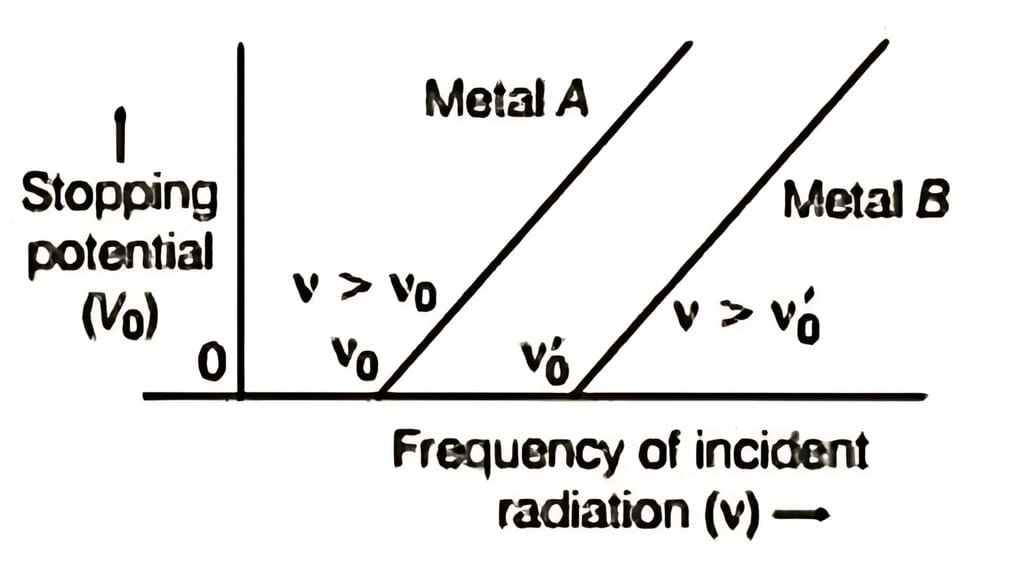 Variation of stopping potential versus frequency of incident radiation
Variation of stopping potential versus frequency of incident radiation
Linear Relationship: For a given photosensitive material, the stopping potential V0 varies linearly with the frequency of incident radiation.
Threshold Frequency: There is a minimum cut-off frequency ν0 below which no photoelectric emission occurs, regardless of intensity. This cut-off frequency is called the threshold frequency.
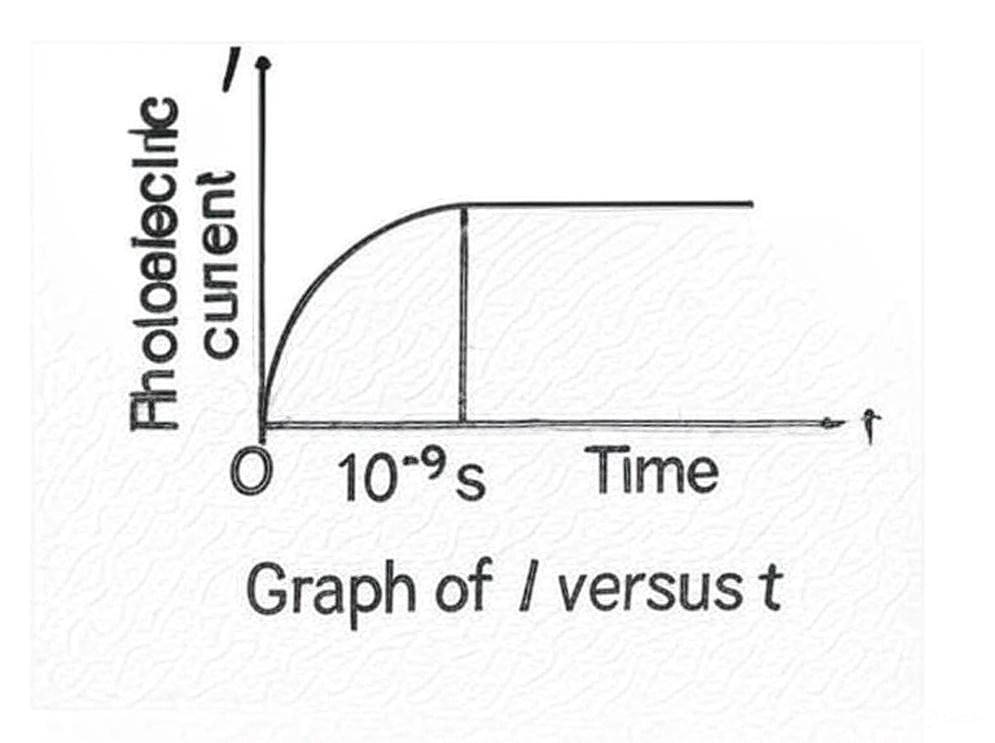
Note:The minimum frequency required to emit photoelectrons is known as the threshold frequency or cut-off frequency and is a characteristic of each material. Below this frequency, no photoelectric emission takes place, even if the light intensity is high. If the frequency is above the threshold, photoemission occurs immediately (within 10−9 seconds or less), even with low-intensity light.
Laws of Photoelectric Emission
- Proportionality with Intensity: For a given material and a fixed frequency of incident radiation, the photoelectric current (or the rate at which photoelectrons are emitted) is directly proportional to the intensity of the incoming light.
- Saturation Current and Stopping Potential: For a given material and frequency of incoming light, the saturation current increases with the intensity of the radiation. However, the stopping potential remains unaffected by the light's intensity.
- Threshold Frequency: Each material has a minimum frequency of incoming radiation, known as the threshold frequency. Below this frequency, no photoelectrons are emitted, regardless of how intense the light is. When the frequency is above the threshold, the maximum energy of the photoelectrons depends only on the frequency (or wavelength) of the light, not its intensity.
- Instantaneous Emission: The emission of photoelectrons occurs almost instantaneously. The time delay between the arrival of radiation and the emission of photoelectrons is extremely short, less than 10-9 seconds.
Photoelectric Effect And Wave Theory of Light
- Light has been a subject of fascination for thinkers and scientists throughout history.
- It was only in the late 17th century that people started to understand the properties of light.
- Sir Isaac Newton proposed the idea that light consists of tiny particles called photons.
- In contrast, Christian Huygens argued that light behaves like waves moving perpendicular to their direction.
- In 1678, Huygens introduced the concept that every point where light is disturbed acts as a source of a new spherical wave.
- The overall shape of this wave results from the combination of all the secondary waves generated by the disturbance.
- This idea is known as Huygens' Principle.
- Using this principle, Huygens successfully derived the laws of reflection and refraction of light.
- He also clarified how light travels in straight lines and in spherical patterns.
- However, Huygens faced challenges in explaining the diffraction effects of light.
- In 1803, Thomas Young conducted an experiment on light interference that supported Huygens' wave theory.
- Later, in 1815, Fresnel provided mathematical equations that supported Young's findings.
- Max Planck 's research introduced the idea that light can exist in small energy packets called photons, which are related to the frequency and speed of light.
- Then, in 1905, Albert Einstein proposed that light exhibits both particle and wave characteristics, suggesting that light is made up of tiny particles called photons.
- This dual nature of light was later confirmed by the field of quantum mechanics.
Energy Quantum of Radiation
Photoelectric Effect refers to the phenomenon where electrons are emitted from a metal surface when it is exposed to light of sufficient energy. This effect was first discovered by Heinrich Hertz in 1887 and later investigated by Lenard in 1902. However, these observations could not be adequately explained by Maxwell's theory of electromagnetic waves at the time.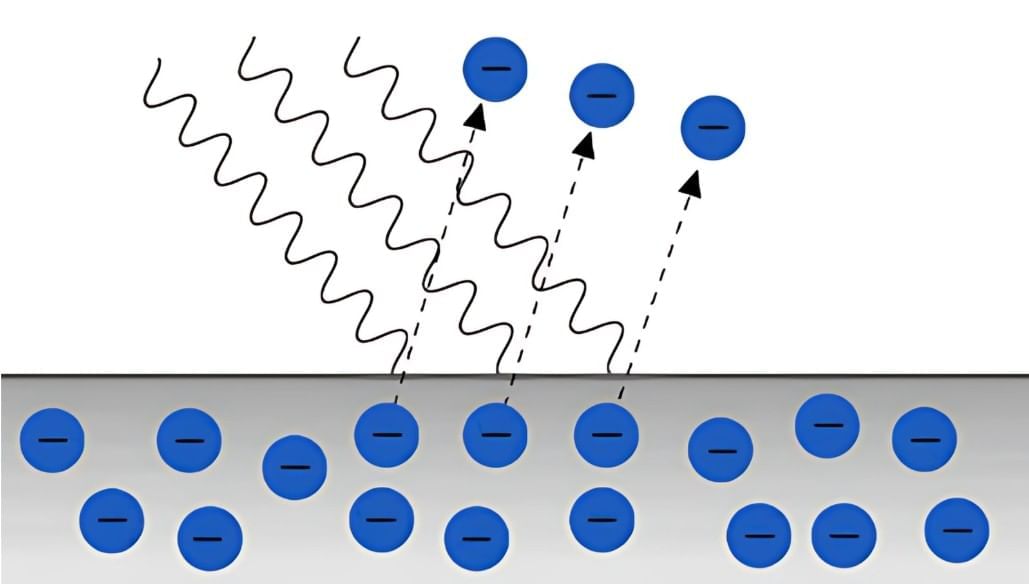
Hertz, who supported the wave theory, did not pursue further research, believing that existing theories were sufficient. However, several challenges to the wave theory emerged, which are summarized below:
- Wave theory posits that energy is distributed evenly across the wavefront and depends solely on the intensity of the light beam. According to this theory, the kinetic energy of emitted electrons should increase with higher light intensity. However, experiments demonstrated that kinetic energy was not affected by intensity.
- Wave theory suggests that any frequency of light should be capable of releasing electrons. In reality, electron emission occurs only at frequencies above a certain threshold frequency, known as ν0 .
- Since wave theory claims that energy is dependent on intensity, low-intensity light should take time to accumulate enough energy to emit electrons. Contrary to this expectation, electron emission was found to be instantaneous, regardless of the light's intensity.
Einstein’s Explanation of Photoelectric Effect
- Einstein resolved this problem using Planck’s revolutionary idea that light was a particle.
- The energy carried by each particle of light (called quanta or photon) is dependent on the light’s frequency (ν) as shown:
E = hν
where h = Planck’s constant = 6.6261 × 10-34 Js. - Since light is bundled up into photons, Einstein theorized that when a photon falls on the surface of a metal, the entire photon’s energy is transferred to the electron.
- A part of this energy is used to remove the electron from the metal atom’s grasp and the rest is given to the ejected electron as kinetic energy.
- Electrons emitted from underneath the metal surface lose some kinetic energy during the collision. But the surface electrons carry all the kinetic energy imparted by the photon and have the maximum kinetic energy.
- We can write this mathematically as:
Energy of photon = energy required to eject an electron (work function) + Maximum kinetic energy of the electron
E = W + K.E.
hv = W + K.E.
K.E. = hv – w - At the threshold frequency, ν0 electrons are just ejected and do not have any kinetic energy. Below this frequency, there is no electron emission. Thus, the energy of a photon with this frequency must be the work function of the metal.
w = hv0 - Thus, Maximum kinetic energy equation becomes:
KE = 1 / 2mv2max = hv – hv0
1 / 2mv2max = h(v − v0) - Vmax is the maximum kinetic energy of the electron. It is calculated experimentally using the stopping potential.
Stopping potential = ev0 = 1 / 2mv2max - Thus, Einstein explained the Photoelectric effect by using the particle nature of light.
Verification of Laws of Photoelectric Emission Based on Einstein’s Photoelectric Equation
Einstein’s photoelectric equation is: 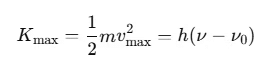
This equation effectively explains the laws of photoelectric emission:
Threshold Frequency Requirement: If the frequency ν is less than the threshold frequency ν0, then
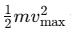 would be negative, which is impossible. Therefore, for photoelectric emission to occur, the frequency ν must be greater than ν0.
would be negative, which is impossible. Therefore, for photoelectric emission to occur, the frequency ν must be greater than ν0.Proportionality with Intensity: Since each photon causes the release of one electron, the number of emitted photoelectrons per second is directly proportional to the intensity of the incident light.
Kinetic Energy and Frequency: The maximum kinetic energy
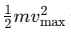 is directly proportional to the frequency ν, as h and ν0 are constants. This shows that the kinetic energy of the photoelectrons increases with the frequency of the incident light.
is directly proportional to the frequency ν, as h and ν0 are constants. This shows that the kinetic energy of the photoelectrons increases with the frequency of the incident light.Instantaneous Emission: Photoelectric emission is the result of elastic collisions between photons and electrons, meaning there is no significant time delay between the photon hitting the surface and the emission of a photoelectron.
Graphs Related to Photoelectric Effect From Einstein's Photoelectric Equation
The important graphs related to the photoelectric effect are as follows:
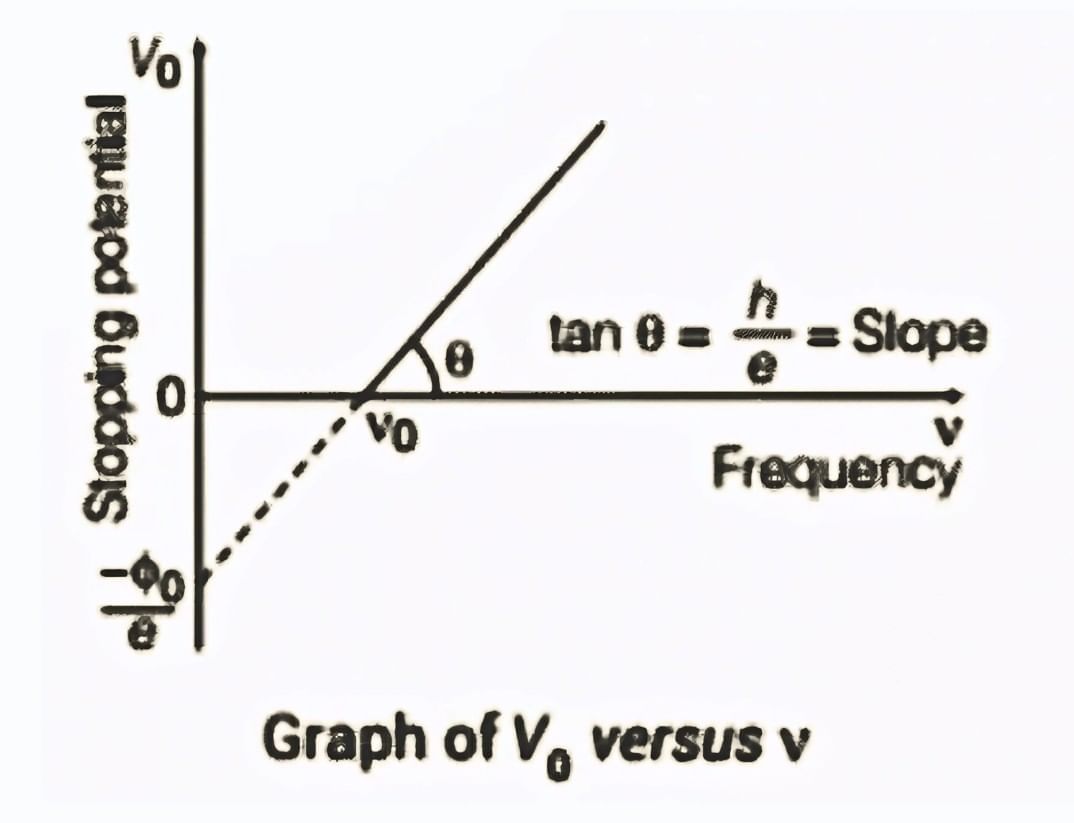
Frequency ν and Stopping Potential Vo Graph
We know that,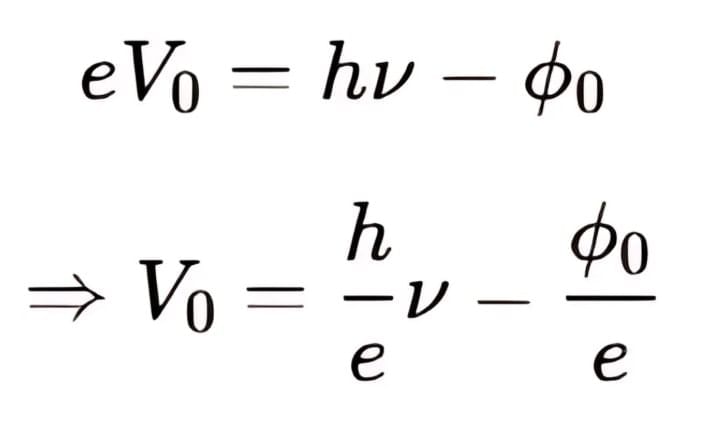 So,
So, 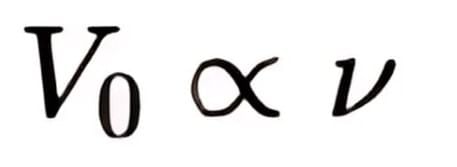
It can be seen that, Vo versus ν curve is a straight line with slope = h/e and is independent of the nature of the material.
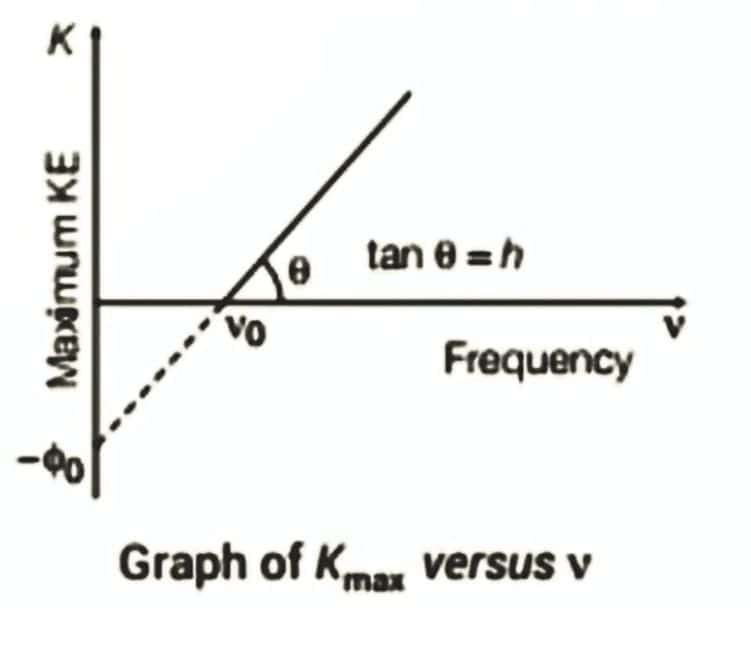
Frequency ν and Maximum Kinetic Energy Graph
As,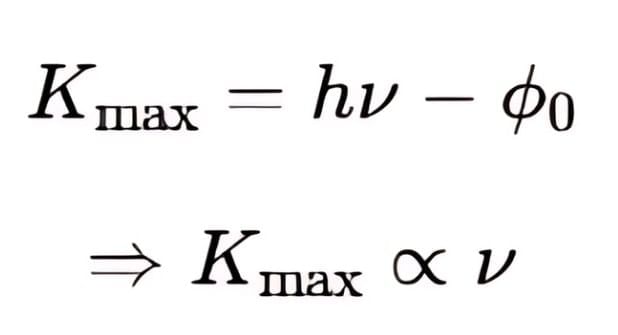
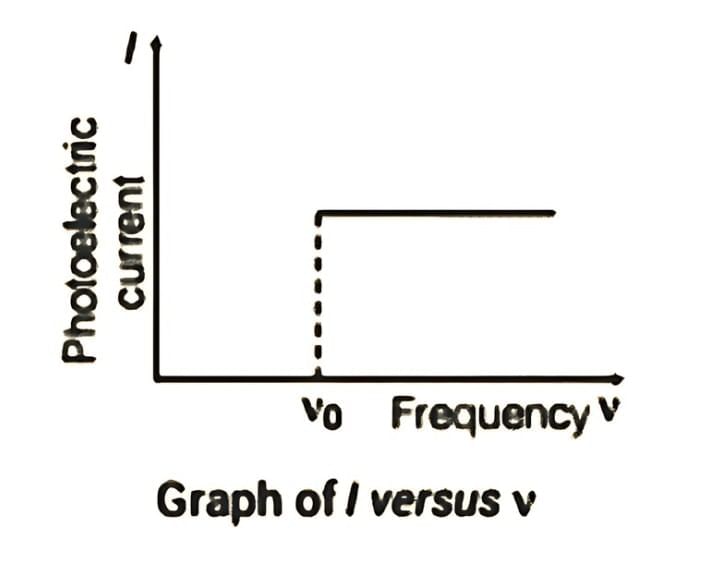
Frequency ν and Photoelectric Current I Graph
The graph below shows that the photoelectric current I is independent of the frequency of the incident light, provided the intensity remains constant.
4. Intensity and Stopping Potential Vo Graph
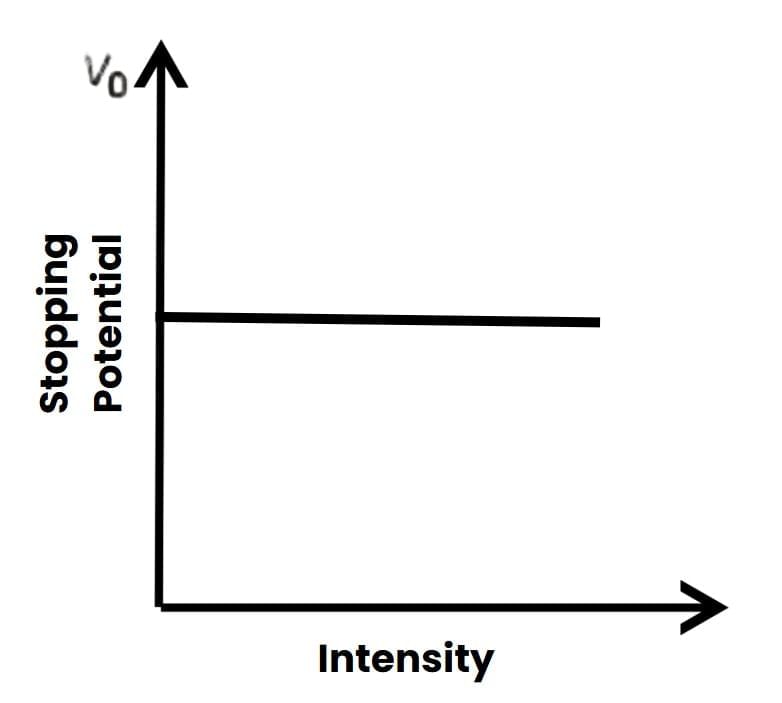 Graph of Vo versus Intensity5. Photoelectric Current I and Time lag t Graph
Graph of Vo versus Intensity5. Photoelectric Current I and Time lag t Graph
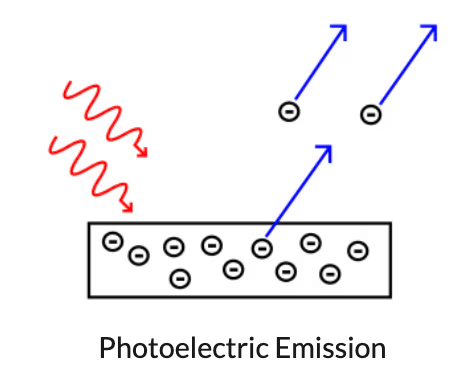
Particle Nature of Light: The Photon
The emission of free electrons from a metal surface when the light is shone on it, it is called the photoemission or the photoelectric effect.
- This effect led to the conclusion that light is made up of packets or quantum of energy.
- Now the question was whether the light quantum theory was indicative of the particle nature of light.
- Einstein already associated the light quantum with momentum.
- This strongly supported the particle nature of light and these particles were named photons.
- Thus, the wave-particle duality of light came into the picture.
Some points to be kept in mind are:
- A photon is a fundamental particle that represents a quantum of light.
- The energy of a photon is calculated using the formula E = hν, where E is energy, h is Planck's constant, and ν is the frequency of light. Its momentum is given by p = h / λ, where λ is the wavelength of light, and its speed is c, the speed of light.
- Regardless of the intensity of radiation, every photon of a given frequency ν has the same momentum p = h / λ and energy E = hν.
- Increasing light intensity means more photons are passing through a specific area per unit time, but it does not change the energy of the radiation.
- A photon is not affected by electric and magnetic fields because it is electrically neutral.
- Photons have zero mass, making them massless particles.
- They are stable particles, and photons can be created or destroyed during the processes of radiation emission or absorption.
- Energy and momentum are conserved during a collision between a photon and an electron.
- A photon cannot decay on its own, but its energy can be transferred during interactions with other particles.
- Photons are spin-1 particles, which is different from electrons that have a spin of ½. The spin axis of a photon aligns with its direction of travel, which is why light can be polarized.
Wave Nature of Matter
- The wave nature of matter is a concept in physics that can be hard to understand because it goes against our intuition.
- To grasp this, think about the diffraction experiment and how it produces interference rings, which show light's wave nature, much like how ripples in a pond interact with each other.
- On the other hand, the photoelectric effect, explained by Albert Einstein, demonstrates that electrons and photons can behave like particles, similar to a billiard ball. This duality is not only intriguing but also connects to how we see things.
- The lens of our eye gathers and focuses light, showcasing its wave properties. However, when photons hit the rods and cones in our retina, they act like particles.
- While we were trying to make sense of this puzzling behavior, Louis de Broglie contributed to the discussion with his de Broglie Relation, adding another layer of complexity to the understanding of matter's wave nature.
De Broglie’s Equation
- De Broglie’s hypothesis stated that there is symmetry in nature and that if light and radiation behave as both particles and waves, matter too will have both the particle and wave nature.
- λ = h / p = h / mv
- Through the de Broglie’s relationship, we now had a wave theory of matter. The ‘Lambda’ here represents the wavelength of the particle and p represents the momentum of the particle.
- The significance of the de Broglie relationship is that it proves mathematically that matter can behave as a wave. In layman terms, the de Broglie equation says that every moving particle – microscopic or macroscopic – has its own wavelength.
- For macroscopic objects, the wave nature of matter is observable.
- For larger objects, the wavelength gets smaller with the increasing size of the object, quickly becoming so small as to become unnoticeable which is why macroscopic objects in real life don’t show wave-like properties.
- Even the cricket ball you throw has a wavelength that is too small for you to observe. The wavelength and the momentum in the equation are connected by the Plank’s constant.
Heisenberg’s Uncertainty
- The Davisson-Germer experiment proved beyond doubt the wave nature of matter by diffracting electrons through a crystal.
- In 1929, de Broglie was awarded the Nobel Prize for his matter wave theory and for opening up a whole new field of Quantum Physics.
- The matter-wave theory was gracefully incorporated by Heisenberg’s Uncertainty Principle.
- The Uncertainty Principle states that for an electron or any other particle, both the momentum and position cannot be known accurately at the same time.
- There is always some uncertainty with either the position ‘delta x’ or with the momentum, ‘delta p’.
- Heisenberg’s Uncertainty Equation: delta x . delta p ≤ h / 2
Say you measure the momentum of the particle accurately so that ‘delta p’ is zero. To satisfy the equation above, the uncertainty in the position of the particle, ‘delta x’ has to be infinite.
From de Broglie’s equation, we know that a particle with a definite momentum has a definite wavelength ‘Lambda’. A definite wavelength extends all over space all the way to infinity. By Born’s Probability Interpretation, this means that the particle is not localized in space and therefore the uncertainty of position becomes infinite.
In real life though, the wavelengths have a finite boundary and are not infinite and thus both the position and momentum uncertainties have a finite value. De Broglie’s equation and Heisenberg’s Uncertainty Principle are apples of the same tree.
Some Solved Examples -
Example 1. The photoelectric threshold of the photoelectric effect of a certain metal is 2750 Å. Find
(i) The work function of emission of an electron from this metal,
(ii) Maximum kinetic energy of these electrons,
(iii) The maximum velocity of the electrons ejected from the metal by light with a wavelength of 1800 Å.
Sol. (i) Given that the threshold wavelength of a metal is λth = 2750 Å. Thus work function of metal can be given as φ = 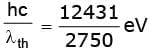
(ii) The energy of an incident photon of wavelength 1800 Å on metal in eV is 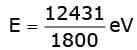 = 6.9 eV
= 6.9 eV
Thus maximum kinetic energy of ejected electrons is
KEmax = E – φ = 6.9 - 4.52 eV = 2.38 eV
(iii) If the maximum speed of ejected electrons is vmax then we have 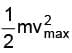 = 2.38 eV
= 2.38 eV
or  = 9.15 X 105 m / s
= 9.15 X 105 m / s
Example 2. Light quanta with an energy of 4.9 eV eject photoelectrons from metal with work function of 4.5 eV. Find the maximum impulse transmitted to the surface of the metal when each electron flies out.
Sol. According to Einstein's photoelectric equation
E = 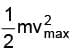 = hv - φ = 4.9 - 4.5 = 0.4 eV
= hv - φ = 4.9 - 4.5 = 0.4 eV
If E be the energy of each ejected photoelectron momentum of electrons is p = 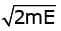
We know that change of momentum is impulse. Here the whole momentum of the electron is gained when it is ejected out thus impulse on the surface is Impulse = 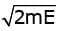
Substituting the values, we get
Maximum impulse = 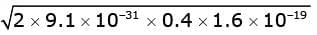 = 3.45 X10 -25 kg m / sec.
= 3.45 X10 -25 kg m / sec.
Example 3. In an experiment tungsten cathode which has a threshold of 2300 Å is irradiated by the ultraviolet light of wavelength 1800 Å. Calculate
(i) Maximum energy of emitted photoelectron and
(ii) Work function for tungsten
(Mention both the results in electron-volts)
Given Plank's constant h = 6.6 X 10 -34 joule-sec, 1 eV = 1.6 X 10 -19joule and velocity of light c = 3 X 108 m/sec.
Sol. The work function of tungsten cathode is 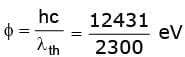 = 5.4 eV
= 5.4 eV
The energy in eV of incident photons is
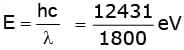
The maximum kinetic energy of ejected electrons can be given as
KEmax = E - φ = 6.9 - 5.4 eV = 1.5 eV
Example 4. Light of wavelength 1800 Å ejects photoelectrons from a plate of a metal whose work functions is 2 eV. If a uniform magnetic field of 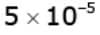 tesla is applied parallel to the plate, what would be the radius of the path followed by electrons ejected normally from the plate with maximum energy.
tesla is applied parallel to the plate, what would be the radius of the path followed by electrons ejected normally from the plate with maximum energy.
Sol. Energy of incident photons in eV is given as
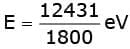
As work function of metal is 2 eV, the maximum kinetic energy of ejected electrons is KEmax = E - φ = 6.9 – eV = 4.9 eV
If vmax be the speed of fastest electrons then we have 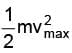 = 4.9 X1.6 X10 -19 joule
= 4.9 X1.6 X10 -19 joule
or 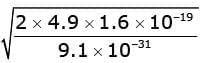 = 1.31 X 106 m/s
= 1.31 X 106 m/s
When an electron with this speed enters a uniform magnetic field normally it follows a circular path whose radius can be given by
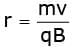
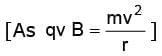
or 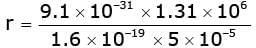 or r = 0.149 m
or r = 0.149 m
|
74 videos|314 docs|88 tests
|
FAQs on Dual Nature Of Radiation And Matter - Physics Class 12 - NEET
| 1. What is electron emission and why is it important in physics? |  |
| 2. What are the main types of electron emission? |  |
| 3. What is the photoelectric effect and how does it demonstrate the particle nature of light? |  |
| 4. What are the laws of photoelectric emission? |  |
| 5. What is Einstein’s photoelectric equation and what does it signify about the dual nature of radiation? |  |

















