Electron transport chain, Oxidative phosphorylation & Photorespiration | Botany Optional for UPSC PDF Download
Mitochondria
 Mitochondria are the energy factories of eukaryotic cells, playing a crucial role in generating adenosine triphosphate (ATP), the cell's primary source of energy. Within these organelles, an intricate system known as the Electron Transport System (ETS) operates. This article explores the components and mechanisms of the Electron Transport System, shedding light on why electrons flow in a particular direction, ultimately contributing to ATP production.
Mitochondria are the energy factories of eukaryotic cells, playing a crucial role in generating adenosine triphosphate (ATP), the cell's primary source of energy. Within these organelles, an intricate system known as the Electron Transport System (ETS) operates. This article explores the components and mechanisms of the Electron Transport System, shedding light on why electrons flow in a particular direction, ultimately contributing to ATP production.
Electron Transport System
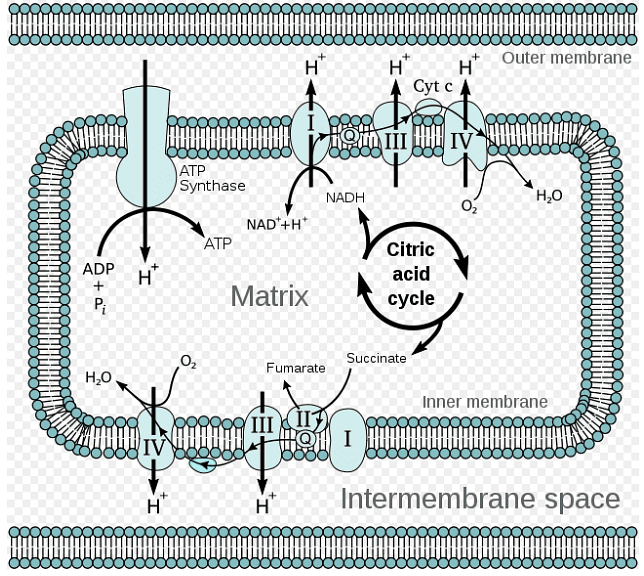 In this diagram, we observe the movement of electrons as they travel from NADH to O2, aided by various intermediary electron carriers. For instance, electrons shift from a reduced source, like malate, to an oxidized source, such as OAA. These electrons progress through a series of carriers, riding a beneficial energy gradient that leads them to the ultimate electron receptor, O2. This favorable gradient arises because electrons gravitate towards components with a more positive reduction potential, indicating a stronger attraction to oxygen. Simultaneously, as electron flow occurs, protons traverse the inner membrane and enter the intermembrane space.
In this diagram, we observe the movement of electrons as they travel from NADH to O2, aided by various intermediary electron carriers. For instance, electrons shift from a reduced source, like malate, to an oxidized source, such as OAA. These electrons progress through a series of carriers, riding a beneficial energy gradient that leads them to the ultimate electron receptor, O2. This favorable gradient arises because electrons gravitate towards components with a more positive reduction potential, indicating a stronger attraction to oxygen. Simultaneously, as electron flow occurs, protons traverse the inner membrane and enter the intermembrane space.
Components of the Electron Transport Chain
The electron transport chain consists of four protein complexes that catalyze the redox reactions involved in the flow of electrons from NADH to O2.
These complexes are:
- Complex I, also known as the NADH-Q reductase complex, catalyzes the transfer of electrons from NADH to coenzyme Q (Q).
- Complex III, or the Cytochrome c reductase complex, facilitates the transfer of electrons from Q to cytochrome c.
- Cytochrome c is a small protein that carries electrons from Complex III to Complex IV.
- Complex IV, or the Cytochrome c oxidase complex, is responsible for the final transfer of electrons to O2, resulting in the formation of water.
When electrons flow from FADH2 to O2, the electron transport chain follows a slightly different pathway. The complexes involved are:
- Complex II, also known as succinate dehydrogenase, catalyzes the transfer of electrons from FADH2 to coenzyme Q. This complex is unique to the pathway of FADH2 electrons.
- Complex III, the Cytochrome c reductase complex, functions as before by transferring electrons from Q to cytochrome c.
- Cytochrome c carries the electrons from Complex III to Complex IV.
- Complex IV, the Cytochrome c oxidase complex, again facilitates the final transfer of electrons to O2.
It is important to note that when FADH2 electrons enter the electron transport chain, they do so at Complex II, which results in a lower pumping of H+ ions to the intermembrane space. This leads to a lower production of ATP compared to the pathway of NADH electrons.
Why do electrons flow in this direction?
- Electrons flow in a specific direction because of the energy relationships determined by electron affinity and redox voltages.
- The change in "standard" reduction potential, measured at specific conditions, helps determine the direction of electron flow.
- The measurement of redox potential is done by comparing it relative to 1M H+/ saturated H2 gas.
- To measure the electron-transfer potential, an experiment is set up with two electrical terminals, one containing the sample cell solution and the other containing the standard reference cell.
- The electrodes in the two solutions are connected by a voltmeter, which measures the voltage between the terminals.
- Electrons flow from the sample cell to the standard reference cell, and the direction of flow can be determined by observing the voltage measured by the voltmeter.
- If electrons flow from the sample cell to the standard reference cell and have a negative voltage, it indicates that an electrode in the solution is connected to another electrode in the standard reference cell.
- By measuring the voltage between the terminals, the direction of electron flow can be determined.
Relationship between the standard free energy change standard delta G and the reduction potential standard delta E: Standard reduction potentials for some reactions:
Standard reduction potentials for some reactions: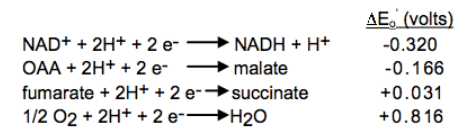 Combine the reactions and standard reduction potentials: Reverse the donor half-reaction and multiply its reduction potential by -1; add the reactions and reduction potentials
Combine the reactions and standard reduction potentials: Reverse the donor half-reaction and multiply its reduction potential by -1; add the reactions and reduction potentials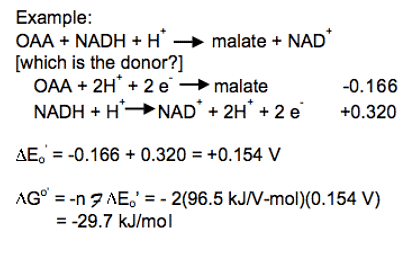 Combine the reactions and standard reduction potentials: Reverse the donor half-reaction and multiply its reduction potential by -1; add the reactions and reduction potentials
Combine the reactions and standard reduction potentials: Reverse the donor half-reaction and multiply its reduction potential by -1; add the reactions and reduction potentials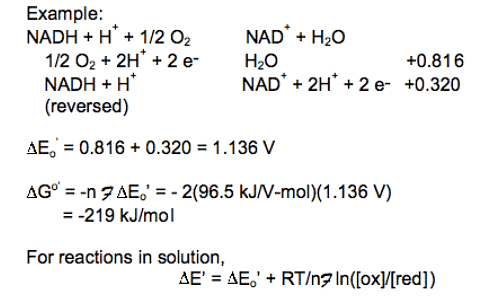 In this reaction a significant amount of energy is released by the reduction of O2 with NADH. The free energy released in this reaction generates the proton gradient that drives the synthesis of ATP and transport of other metabolites.
In this reaction a significant amount of energy is released by the reduction of O2 with NADH. The free energy released in this reaction generates the proton gradient that drives the synthesis of ATP and transport of other metabolites.
What is Oxidative Phosphorylation?
Oxidative phosphorylation represents the third stage of food catabolism, where energy transfer shifts from food to high-energy molecules, particularly energetic electron carriers such as NADH and FADH2. Notably, these electron carriers do not travel in isolation; they simultaneously transport protons (2H+ + 2e−). The journey of electrons in NADH and FADH2 commences with a series of enzymes embedded in the inner mitochondrial membrane, culminating in the reduction of molecular oxygen (O2) to water (H2O) via the following reaction:

At each step of this electron transfer process, electrons move from substances with a higher reduction potential to those with a lower reduction potential, liberating energy proportionate to the reduction potential difference. This energy is then harnessed by complexes I, III, and IV, which pump protons (H+) across the mitochondrial inner membrane, creating an electrochemical potential energy gradient. ATP synthase, a pivotal player in this process, facilitates the flow of protons back into the matrix and harnesses the released energy to synthesize high-energy ATP by phosphorylating low-energy ADP with inorganic phosphate (Pi). Collectively, this intertwined web of electron transport and ATP synthesis is termed oxidative phosphorylation, taking place within the cellular mitochondria.
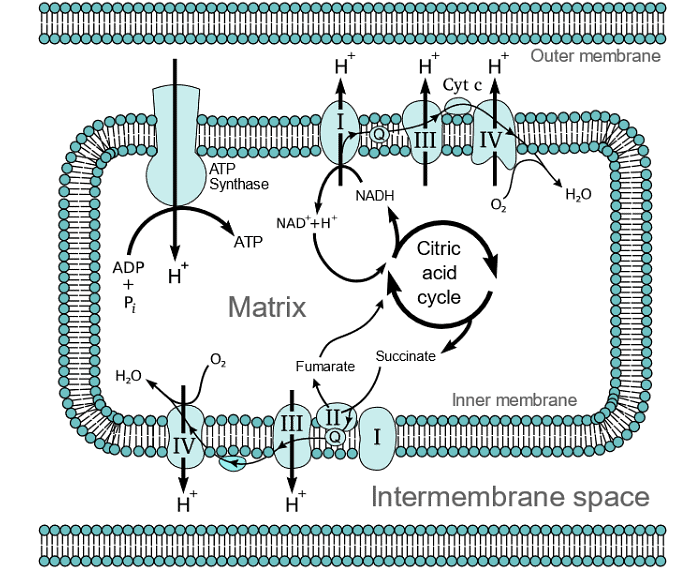
Electron Transport Chain
- In the second part of stage 3 of cellular respiration, electrons are extracted from molecules like NADH and FADH2 and are used to convert oxygen (O2) into water (H2O) through a series of enzyme complexes known as Complex I through IV, as well as mobile electron carriers such as ubiquinone (Q) and cytochrome c (Cytc). This process is called the electron transport chain, where electrons move from substances with higher reduction potential to those with lower reduction potential, releasing energy in the process.
- Complexes I, III, and IV span from the inner mitochondrial matrix to the intermembrane space through the inner mitochondrial membrane. These complexes harness the released energy to pump protons (H+) from the matrix to the intermembrane space, creating an electrochemical gradient. Complex II, on the other hand, is embedded in the inner membrane but does not contribute to proton pumping. Instead, it participates in electron transport. Coenzyme-Q (Q) is a hydrophobic molecule that carries electrons through the lipid bilayer from complexes I and II to complex III. Cytochrome c (Cytc) is a hydrophilic molecule that moves along the inner membrane's surface, transferring electrons from complex III to complex IV. These enzyme complexes and electron carriers play a crucial role in both the electron transport chain and ATP synthesis.
- Complex I, also called NADH-coenzyme Q oxidoreductase, is the initial enzyme complex in the electron transport chain. It starts when NADH binds to complex I and donates two electrons. These electrons are passed through iron-sulfur clusters within complex I to coenzyme-Q (Q), which is reduced to QH2. As the electrons move through complex I, it pumps four protons (4H+) from the matrix to the intermembrane space, establishing a proton gradient across the inner mitochondrial membrane. The exact mechanism of proton pumping is not completely understood, but it is believed to involve conformational changes within complex I. The overall redox reaction in complex I is:
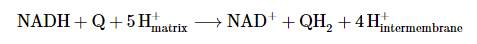
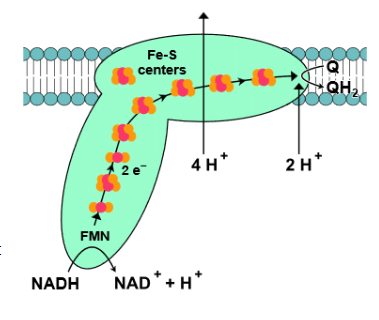
- Complex II, also known as succinate-Q oxidoreductase, provides another entry point for electrons into the electron transport chain. It is part of the citric acid cycle and the electron transport chain. Succinate is oxidized to fumarate in the citric acid cycle, resulting in the conversion of FAD to FADH2 within complex II. Electrons and protons are then transferred from FADH2 through iron-sulfur clusters to coenzyme-Q in complex II. Coenzyme-Q subsequently passes these electrons to complex III.
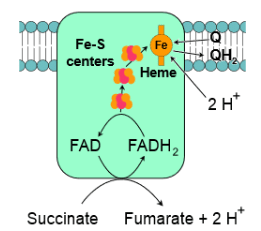
- Notably, FADH2 is less energetically favorable than NADH. Because of this, complex II does not contribute to proton pumping from the matrix to the intermembrane space. Instead, the energy released during electron transport is used for proton pumping in complex III and IV, as explained in the following section.
- Complex III, also called cytochrome C reductase, contains protein subunits, an iron-sulfur cluster, and cytochromes. Cytochromes are proteins with heme groups that can accept and release electrons. Complex III transfers electrons from coenzyme-Q (QH2) to cytochrome c (Cyt c). Since QH2 delivers two electrons while Cyt c can only accept one, this transfer occurs in two steps. In the first step, QH2 transfers one electron to Cyt c and one to another coenzyme-Q in quinone (Q) form, releasing 2H+. Quinone converts to a semiubiquinone (Q∙−). In the second step, another QH2 transfers one electron to a second Cyt c and one to Q∙−, converting it back to Q, which is then released from the complex. This process results in the transfer of two electrons from a Q molecule to two Cyt c molecules, and it pumps 4H+ into the intermembrane space.
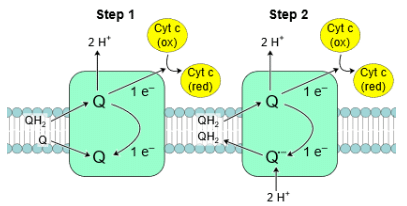
- Complex IV, known as cytochrome c oxidase, is the final complex in the electron transport chain. It contains multiple subunits, two heme groups, and various metal ion cofactors. Complex IV reduces oxygen (O2) into water (H2O) while oxidizing Cyt c. During this process, it pumps 4H+ from the matrix to the intermembrane space. This is a crucial step in generating ATP through oxidative phosphorylation.
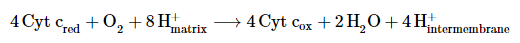
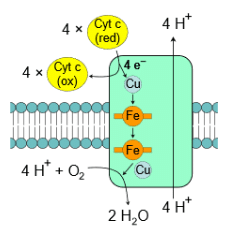
In summary, the electron transport chain is a vital component of cellular respiration, facilitating the transfer of electrons and the pumping of protons across the inner mitochondrial membrane to produce ATP.
ATP Synthase: The ATP Production Hub
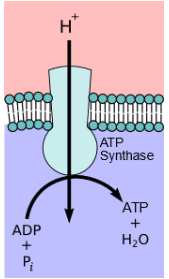
- Complex V, also known as ATP synthase, concludes the oxidative phosphorylation process within stage 3 of food catabolism. It consists of two distinct parts, FO and F1, which work in unison to generate ATP from the electrochemical potential energy harnessed earlier in the process.
- The FO section acts as a proton pore, enabling the movement of protons (H+) across the inner mitochondrial membrane. The resulting electrochemical gradient creates the necessary potential energy. ATP synthase, using this energy, catalyzes the synthesis of ATP.
Chemiosmosis
Chemiosmosis is a fundamental biological process that involves the movement of ions, specifically protons (H+), across a semipermeable membrane down their electrochemical gradient. This process plays a crucial role in cellular energy production, particularly in the synthesis of adenosine triphosphate (ATP), the cell's primary energy currency.
Here's a breakdown of the key concepts related to chemiosmosis:
- Osmosis: Osmosis is the natural movement of water molecules from an area of higher water concentration to an area of lower water concentration through a semipermeable membrane. It occurs in response to differences in solute concentration and aims to equalize the concentration on both sides of the membrane.
- Proton Movement: During cellular respiration, protons (H+) are actively pumped from the mitochondrial matrix to the intermembrane space by enzyme complexes I, III, and IV of the electron transport chain. This proton movement creates a concentration gradient, with a higher concentration of protons in the intermembrane space compared to the matrix.
- Electrochemical Gradient: The movement of protons results in the establishment of an electrochemical gradient across the inner mitochondrial membrane. This gradient consists of both a concentration gradient (due to differing proton concentrations) and an electrical gradient (due to the positive charge of protons). Together, they form an electrochemical potential energy gradient.
- Chemiosmosis: Chemiosmosis is the process by which ions, in this case, protons (H+), flow across a semipermeable membrane down their electrochemical gradient. In mitochondria, this membrane contains ATP synthase, a specialized enzyme.
- ATP Synthase: ATP synthase is an enzyme embedded in the inner mitochondrial membrane. It acts as a molecular turbine or rotor. As protons flow back into the mitochondrial matrix down their electrochemical gradient through ATP synthase, this enzyme harnesses the energy of their movement to synthesize ATP from adenosine diphosphate (ADP) and inorganic phosphate (Pi). The energy is used to phosphorylate ADP, converting it into ATP.
- Oxidative Phosphorylation: Chemiosmosis is a key part of the process known as oxidative phosphorylation. During oxidative phosphorylation, the energy released during the transfer of electrons through the electron transport chain (complexes I, III, and IV) is used to pump protons and create the electrochemical gradient. This energy is then utilized by ATP synthase to generate ATP from ADP and Pi.
- Equilibrium Reaction: The synthesis of ATP via ATP synthase is a reversible reaction. When the electrochemical gradient is low or when there is a surplus of ATP in the cell, ATP synthase can work in reverse, consuming ATP and actively pumping protons from the matrix back into the intermembrane space. This helps maintain the balance of ions and energy in the cell.
Mechanism of ATP Synthesis
ATP synthase comprises multiple protein subunits and is shaped like a mushroom, with FO embedded in the inner membrane and F1 protruding into the matrix. The rotation of F1γ within the F1(αβ)3 complex triggers conformational changes in the β subunits, ultimately catalyzing ATP synthesis. Each complete 360o turn of F1γ generates three ATP molecules from three F1β subunits.
Heating the Body: Energy Dissipation
Intriguingly, the energy released during oxidative phosphorylation contributes to body heating. Certain compounds known as uncouplers, such as 2,4-dinitrophenol and dicumarol, decouple electron transport from ATP synthase. While electrons are transported as usual, protons return to the matrix without ATP production, resulting in the release of energy as heat.
Reactive Oxygen Species (ROS) and Their Management
During this intricate process, there is a risk of reactive oxygen species (ROS) formation, which can be harmful. ROS can damage proteins, cause DNA mutations, and accelerate the aging process. To counteract this, the body employs mechanisms to suppress ROS production and neutralize them when formed. The production of ROS escalates with increasing intermembrane potential.
ATP Yield Comparison: Aerobic vs. Anaerobic Catabolism
Aerobic catabolism, as facilitated by oxidative phosphorylation, yields approximately thirty ATP molecules per glucose molecule. In contrast, anaerobic catabolism, such as fermentation, yields only two ATP molecules per glucose molecule.
Conclusion
Oxidative phosphorylation, an essential component of food catabolism, is a complex and precisely regulated process that generates ATP, provides heat energy, and carries potential risks associated with ROS production. This comprehensive article has delved into the intricacies of this critical biological process, shedding light on its various components and their interconnected roles in energy production and cellular function. Understanding oxidative phosphorylation is crucial for unraveling the mysteries of cellular energy metabolism and its implications for health and disease.
Photorespiration
- Photorespiration is an intricate metabolic process primarily taking place within the leaf cells of plants. Often referred to as a wasteful pathway, photorespiration competes with photosynthesis, exerting negative impacts on plant growth and overall productivity. The initiation of photorespiration is triggered by a low concentration of carbon dioxide (CO2) within the leaf, coupled with a high concentration of oxygen (O2). In this article, we delve into the definition, underlying process, and the significance of photorespiration in the world of plant biology.
- Photorespiration is a metabolic process that occurs concomitantly with the Calvin cycle, and it is also known by the name C2 Cycle. At the heart of this process lies the crucial enzyme known as RuBisCO, which plays a pivotal role in facilitating photorespiration. This metabolic activity predominantly unfolds in C3 plants, where it serves to mitigate the inefficiencies associated with RuBisCO. During the mechanism of photorespiration, oxygen (O2) is consumed while carbon dioxide (CO2) is released in the presence of light. Essentially, photorespiration mirrors the respiration process, as both reactions involve the utilization of O2 and the release of CO2.
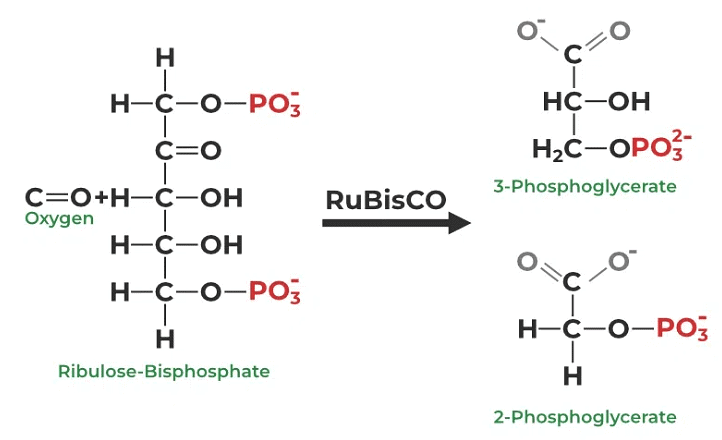
Organelles Involved in Photorespiration
3 cell organelles are involved in photorespiration those are:
- Chloroplast
- Peroxisomes
- Mitochondria
Photorespiration as a Wasteful Process
The initiation of photorespiration hinges upon the oxygenation of ribulose-1,5-bisphosphate-carboxylase/oxygenase (RuBisCO), which is also the enzyme responsible for CO2 fixation in virtually all photosynthetic organisms. Phosphoglycerate, formed in the presence of oxygen, is recycled within the Calvin cycle's intermediate stages in the Photorespiratory pathway. This diversion of reactions consumes energy and ultimately results in the release of previously fixed carbon as CO2. Thus, photorespiration is often viewed as an inefficient process.
Factors Influencing Photorespiration
- Several factors influence the extent of photorespiration in plants, including light concentration.
- In submerged and stressful conditions, higher light concentrations tend to promote greater photorespiration.
Benefits of Photorespiration
While photorespiration is predominantly perceived as wasteful, it does offer certain benefits in the realm of plant biology:
- Plant-Resistant Protections: Photorespiration plays a role in bolstering a plant's resistance mechanisms.
- Maintaining Redox Equilibrium: It contributes to maintaining the redox equilibrium within plant cells.
Significance of Photorespiration
- Photorespiration can be viewed as the antithesis of photosynthesis, as it counters the efficiency of this vital process.
- Importantly, photorespiration does not yield ATP or NADPH, further emphasizing its inefficiency in energy production.
In conclusion, photorespiration, despite its inefficiency, is an essential metabolic process in the world of plant biology, impacting various facets of plant growth and survival. Understanding its mechanisms and significance sheds light on the intricate dance of life within plant cells.
|
179 videos|143 docs
|
FAQs on Electron transport chain, Oxidative phosphorylation & Photorespiration - Botany Optional for UPSC
| 1. What is oxidative phosphorylation? |  |
| 2. What is the electron transport chain? |  |
| 3. How does oxidative phosphorylation differ from photorespiration? |  |
| 4. What are the benefits of oxidative phosphorylation? |  |
| 5. How is oxidative phosphorylation regulated? |  |















