Elimination Reactions and The Zaitsev Rule | Chemistry Optional Notes for UPSC PDF Download
Introduction to Elimination Reactions
- As you’ve probably noticed by now, organic chemistry is a lot different from physics. When we’re looking to predict what reaction might occur in a given situation, we don’t have a handy series of equations we can simply refer to.
- No, it’s messier than that. We take the experimental results, work backwards, and then try to rationalize what is happening in terms of the key concepts at work.
- Furthermore, to use an analogy, organic chemistry isn’t “digital”. It’s extremely uncommon that one set of conditions will give you 100% yield of one product, and a different set of conditions will give you 100% yield of another.
- Rather, organic chemistry is “analog”. Think of each reaction as having a series of knobs we can turn, which can “tune” a reaction toward a different result. (Some examples of “knobs” – temperature, solvent, type of substrate, type of leaving group, type of nucleophile….).
- I say this because this post is the first in a series on elimination reactions, the third of the “four most important” reactions in first semester organic chemistry. Elimination reactions often occur under similar conditions to substitution reactions, which means that we will have to learn how to think about how these reactions compete with each other.
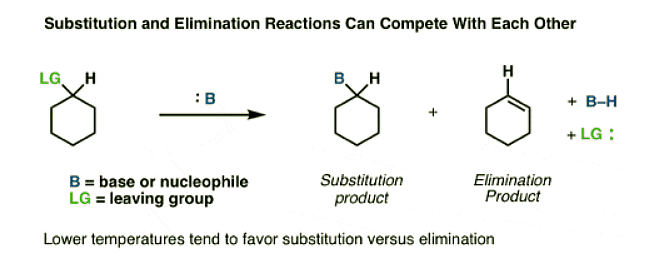
Elimination Reactions Often Accompany Substitution Reactions
Let’s start with a concrete example. In these two reactions, substitution is the major product. However, the yield of substitution products is not 100%. As it turns out there are actually some minor byproducts in this reaction that are not substitution products.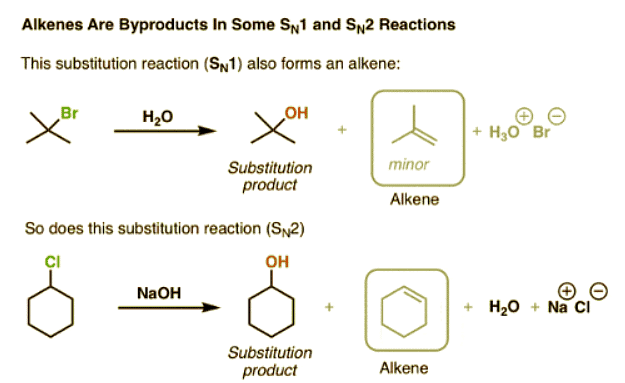
Remember that the key pattern of bond forming/bond breaking in a substitution reaction is a simple swap of a C-(leaving group) bond for a C-(nucleophile) bond? Clearly, in the boxed products, this isn’t occurring here. Let’s break down these patterns in more detail.
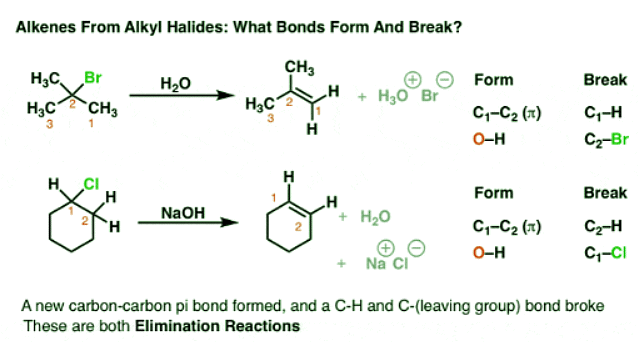
Elimination Reactions: The Key Bond Forming/Breaking Pattern
Note the key pattern here. In each case, we’re forming a new C–C π bond, and breaking two single bonds to carbon. So this is a completely different pattern than acid-base reactions or substitution reactions, because it involves two adjacent carbon atoms. This class of reactions are called “elimination” reactions.
However, it’s not *completely* without comparison to reactions we’ve seen before:
- Note how we’re breaking a C-H bond and forming a new base between H and something else (in the case above, oxygen). We’ve seen this before. This is an “acid-base” reaction. So one component of the elimination reactions we will see is the involvement of a strong base.
- In addition, we’re breaking a bond between carbon and a leaving group. We’re familiar with leaving groups by now; remember how good leaving groups are weak bases? The same principle applies to elimination reactions as well.
What’s slightly new here is that we’re forming a C-C π bond in addition to the above.
So when you add up the number of species in solution, we’re going from two species in solution (base and substrate) to three species (product, conjugate acid, and leaving group).
Elimination Reactions: The Zaitsev Rule
- Elimination reactions usually occur such that they are removing a hydrogen from the carbon attached to the fewest hydrogens.
- This is called “Zaitsev’s rule”.
- So when you form an alkene in an elimination reaction, make sure you form the most substituted alkene (i.e. the one with the most carbon atoms directly attached).
- Another way of stating Zaitsev’s rule is that “the poor get poorer”. In other words, the carbon with the fewest hydrogens loses the hydrogen.
In Elimination Reactions, The “More Substituted” Alkene Tends To Be The Major Product
- So far, we’ve only looked at some simple elimination reactions where only one product is possible. In this post we’ll look at some examples where we start to see some of the extra “wrinkles” that can be present in elimination reactions.
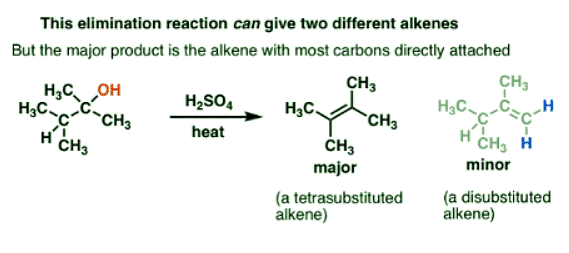
For example, if you heat the alcohol below with a strong acid (like sulfuric acid, H2SO4) you obtain one major product (an alkene) and a minor product (also an alkene).
- What’s interesting about this? Well, if you look closely you should see that actually two elimination products are possible here, but only one is formed as the major product.
- The alkene which is “tetrasubstituted” – that is, attached to four carbon atoms – is the major product, and not the “disubstituted” alkene, which is attached to two carbon atoms and two hydrogen atoms.
- (The fact that we’re forming a new C-C π bond at the expense of sigma bonds on adjacent carbons is characteristic of elimination reactions.)
- Similarly, look at the product of this next reaction. Taking an alkyl bromide and adding a strong base, we again get a “major” product and a “minor product”.

- Again, the major product is “more substituted” than the minor product. Of the 4 atoms directly attached to the alkene in the major product, 3 of them are carbon and 1 is hydrogen. In the minor product, 2 carbon atoms and 2 hydrogen atoms are directly attached to the alkene.
- So what’s responsible for this preference for the “more substituted” alkene in elimination reactions?
The Stability Of Alkenes Increases As C-H Bonds Are Replaced With C–C Bonds
- Well, this correlates nicely with an observation that’s been made regarding the heats of formation of various alkenes. As an alkene becomes more substituted (i.e. more carbons attached, fewer hydrogens attached) it becomes more thermodynamically stable. [This observation comes from measuring the enthalpy of hydrogenation for various alkenes – click here for data].

- This agrees nicely with the trend that’s observed for elimination reactions. The major product of an elimination reaction tends to be the more substituted alkene. This is because the transition state leading to the more substituted alkene is lower in energy and therefore will proceed at a higher rate.
The “Zaitsev Rule”: Elimination Will Occur Such That The Hydrogen Is Removed From The Beta-Carbon With The Fewest Hydrogens
- It was a Russian chemist named Alexander Zaitsev who published a paper making this observation back in the late 19th century, and therefore this observation has become known as Zaitsev’s Rule. Formally, the rule is that an elimination will occur such that a hydrogen is removed from the “β-carbon” with the fewest hydrogens.
- [Organic chemists and their terms: the “α-carbon” is the carbon attached to the leaving group, while “β-carbons” are all carbons attached to the alpha carbon.]

Elimination Reactions Are Favored By Heat
- Elimination reactions are often in competition with substitution reactions
- Generally speaking, adding heat tends to increase the proportion of elimination products relative to substitution products
- The reason is that elimination results in a greater number of species in solution, which increases entropy, (ΔS) and increasing temperature T makes the –TΔS term in the Gibbs free energy equation, larger.
All Else Being Equal, Elimination Reactions Are Favored Over Substitution Reactions With Increasing Heat
- A few posts back we saw how elimination reactions are often in competition with substitution reactions.
- How do we know when one reaction pathway is going to be preferred over another? As we’ll see, there are going to be several components to answering this question fully, but today we’ll talk about one simple rule of thumb going forward.
- Let’s say you have a reaction like this one. It’s possible for substitution or elimination products to be formed. [I’m keeping the identity of the base and substrate vague here.

- As temperature is increased, the relative amount of elimination products will increase relative to substitution products. You can imagine it looking like this.

Heating Results In A Gradual Increase In Elimination Versus Substitution
- Notice again how organic chemistry works. It’s not as if applying heat is an on/off switch that results in a reaction going from 100% substitution to 100% elimination. Instead, increasing temperature results in a gradual increase in elimination products relative to substitution. That’s because temperature is gradually leading to an increase in the rate constant for elimination versus rate constant for substitution.
- So what’s going on here?
- Here’s one thing we can say with confidence: at low temperatures, the activation energy for the substitution reaction is lower than that for the elimination reaction. Remember that the lower the activation energy, the higher the rate of the reaction. This might help to explain our product distribution: as we increase the temperature, more energy is available, so that the starting materials can ascend the activation barrier to provide elimination reactions also. This fits with what is observed.
- However, there’s an even more fundamental reason why we might see more elimination products as heat is increased, and it has to do with some properties we know about thermodynamics that make rate constants (and activation energies) temperature dependent.
Elimination Reactions Result In An Increase In The Number Of Species In Solution; Substitution Reactions Do Not
Let’s look again at the (generic) reactions:
What do we notice here? Notice that the substitution reaction we’re going from 2 species in the starting material to 2 species in the product. But in the elimination reaction, we’re going from 2 species in the starting material to 3 species in the product. An increase!
Increasing Number Of Species In Solution Means Greater Entropy, Which Means That The –TΔS Term In The Gibbs Equation Increases In Value As Temperature (T) Is Increased
- Since we’re birthing a new species in solution here, that’s going to result in an increase in entropy. And if you think way back to general chemistry, the Gibbs equation told us this relationship:
ΔG = ΔH – T ΔS - Remember that the more negative ΔG is, the more favorable the reaction. As temperature increases, that TΔS term is going to start getting bigger and bigger; this will make Δ G more and more negative. At some point, as temperature is increased, the ΔG for elimination will become more negative than delta G for substitution. In other words, more favorable.

- One thing to be careful about though! When we’re discussing ΔG, we really should be talking about the ΔG of the transition state, not that of the final product. [Why not? Because the stability of products isn’t related to reaction rate (if it was, our bodies would have combusted to CO2 and H2O a long time ago!)]. We give a special designation to thermodynamic terms of the transition state – we put a little double-dagger on them. Like this: ΔG‡ . This “Gibbs energy of activation” is how we define the activation energy of a reaction.
- So you can see by analyzing this term that activation energy can change with temperature!
- At low temperatures, the Gibbs energy of activation for substitution (ΔG‡) is lower in energy (more negative) than that for elimination. But at high temperatures, the Gibbs energy of activation ( ΔG‡ ) for elimination starts to be lower in energy than that for substitution reactions, and hence we get an increase in the amount of elimination product.
ΔG‡ = ΔH‡–TΔS‡ - Again, the bottom line is that, all else being equal, heat will tend to favor elimination reactions.
FAQs on Elimination Reactions and The Zaitsev Rule - Chemistry Optional Notes for UPSC
| 1. What is the Zaitsev rule in elimination reactions? |  |
| 2. Why are elimination reactions favored by heat? |  |
| 3. How does the stability of alkenes change as C-H bonds are replaced with C-C bonds? |  |
| 4. What is the key bond forming/breaking pattern in elimination reactions? |  |
| 5. Are elimination reactions favored over substitution reactions with increasing heat? |  |

|
Explore Courses for UPSC exam
|

|