Equilibria: Types & Relation Between K, Q & G | Chemistry Class 11 - NEET PDF Download
Types of Equilibria
- We encounter so many reactions around us in our day-to-day lives; the rusting of iron, the burning of paper, the souring of curd, the formation of ozone, etc. Many of these reactions involve the presence of components in different phases, such as solid iron coming in contact with gaseous oxygen to form solid iron oxide, which we know as rust.
- Similarly, gaseous hydrogen combines with gaseous oxygen to form liquid water. Dealing with such reactions is a tedious task. If the components are in the same phase, the interaction of the components can be understood easily, while when the components are in different phases, the interaction gets more complicated.
- To simplify the problems and understand the concept, we divide such reactions into different categories, namely, the homogeneous reactions, where the components involved in the reaction are present in the same phase, and the heterogeneous reactions, where the components involved are present in different phases. The methods of dealing with both reactions are different and so is the determination of the equilibrium state.
Homogeneous Equilibria
Let us consider a homogeneous system, given by the following reaction
N2(g) + 3H2(g) ↔ 2NH3(g)
Here, we see that gaseous nitrogen reacts with gaseous hydrogen to form gaseous ammonia. Let us now calculate the equilibrium constant for the above reaction.
In terms of the molar concentration of the components in the reaction, we can write
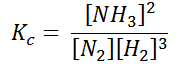 As we know, for reactions involving gases, we express the equilibrium concentration in terms of the partial pressure.
As we know, for reactions involving gases, we express the equilibrium concentration in terms of the partial pressure.
Using the ideal gas equation,
PV = nRT
Where P is the pressure of the system, V is the volume of the system, n is the number of moles of components present in the system, R is the universal gas constant and T is the temperature of the system.
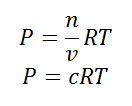
Here, c is the concentration of the system. We can also write it as,
c = P / RT
So equilibrium constant can also be written as,
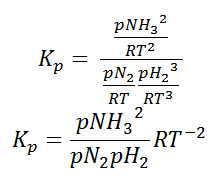
Here, we note that the general expression for Kc and Kp can be written as,
KP = Kc(RT)Δn
Where Δn is defined as the number of moles of gaseous products – the number of moles of gaseous reactants.
N2(g) + 3H2(g) -> 2NH3(g)
Heterogeneous Equilibria
- When the state of equilibrium in a system has components in more than one phase it is termed as a heterogeneous equilibrium.
- For example, if we take a container with ice and water at a temperature that allows the existence of both phases simultaneously, such that, both ice and water are present in a state of equilibrium. This state is termed a heterogeneous equilibrium.
In terms of its equation, this can be written as:
H2O(s) ↔ H2O(l) - In another example, we can consider an aqueous solution of a solid such as calcium hydroxide. We notice that the solid calcium hydroxide is in equilibrium with its saturated solution.
Writing the equilibrium constant for heterogeneous reactions is different from that of the homogeneous reactions. - For example, consider the thermal dissociation of calcium carbonate into calcium oxide and carbon dioxide. Here, we can see that the equilibrium constant for the dissociation of the reactant into its products is only dependent on the gaseous component, as the solid and the liquid reactants are considered to be constant.
CaCO3(s) ↔ CaO(s) + CO2 - Here, the components CaCO3 and CaO are solids, so their molar concentration remains constant throughout the reaction. Therefore, the equilibrium constant can be written as,
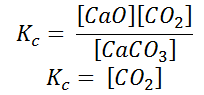
Also, in terms of Kp, we can write
Kp = pCO2
Where p denotes the partial pressure. - In other words, we can state that, at a given temperature, there is a constant concentration or partial pressure of CO2 in the equilibrium reaction with CaO and CaCO3.
Applications of Equilibrium Constant
Equilibrium Constant For Predicting the Extent of Reaction
The equilibrium constant (Kc) can be used to predict the extent of a reaction, i.e. the degree of the disappearance of the reactants. The magnitude of the equilibrium constant gives an idea of the relative amount of the reactants and the products.
Case 1: The larger value of the equilibrium constant (>103) shows that forward reaction is favored i.e. the concentration of products is much larger than that of the reactants at equilibrium.
For Example:
- H2(g) + Br2(g) ⇌ 2HBr(g) ⇒ Kc = 5.4 × 1018
- H2(g) + Cl2(g) ⇌ 2HCl(g) ⇒ Kc = 4 × 1031
- H2(g) + 12O2(g) ⇌ H2O(g) ⇒ Kc = 2.4 × 1047
This shows that at equilibrium, the concentration of the products is very high, i.e. reaction goes almost to completion.
Case 2: The intermediate value of the equilibrium constant (10-3 to 103) shows that the concentration of the reactants and products are comparable.
For Example:
- Fe3 (aq) + SCN (aq) ⇌ [Fe(SCN)]2 (aq) ⇒ Kc = 138 at 298 K
- H2 (g) + I2 (g) ⇌ 2HI (g) ⇒ Kc = 57 at 700 K.
Case 3: Low value of equilibrium constant (<10-3) shows that backward reaction is favored i.e. concentration of reactants is much larger than that of products i.e. the reaction proceeds to a very small extent in the forward direction.
For Example:
- N2 (g) + O2 (g) ⇌ 2NO (g) ⇒ Kc = 4.8 × 10-31 at 298K
- H2O (g) ⇌ H2 (g) + (1/2) O2 (g) ⇒ Kc = 4.1 × 10-48
Equilibrium Constant for Predicting the Direction of a Reaction
The equilibrium constant can be used to predict the direction of the reaction. We need a term, reaction quotient (Qc expressed in terms of concentrations or Qp in terms of partial pressures) similar to the equilibrium constant except that the conditions are not at equilibrium.
For a balanced reaction, aA + bB ⇌ cC + dD
The reaction quotient (Qc or Qp) is given as:
Qc = [C]c[D]d / [A]a[B]b
Qp = pcC × pdD / paA × pbB
Comparison with Kc and the direction of Reaction:
- If Q = Kc, the reaction is in equilibrium [Where, Kc = equilibrium constant]
- If Q > Kc, Q will tend to decrease to become equal to K. As a result, the reaction will proceed in the backward direction.
- If Q < Kc, Q will tend to increase to become equal to K. As a result, the reaction will proceed in the forward direction.
Calculating the Equilibrium Concentration
From Equilibrium Constant
The degree of dissociation of an equilibrium involving gas can be calculated by knowing the equilibrium constant and the concentration of the gaseous reactant/product.
For example, in the decomposition of carbonate, the number of moles of carbon dioxide can be calculated from the equilibrium constant by assuming an ideal gas behavior.
CaCO3 ⇌ CaO + CO2 Kp = [pco2]
The decomposition of ammonia gas:
 Number of moles at equilibrium = C(1 – 2α) + Cα + 3Cα = C(1 + 2α)
Number of moles at equilibrium = C(1 – 2α) + Cα + 3Cα = C(1 + 2α)
Partial pressure of ammonia = C(1 – 2α) / C(1 + 2α) = [(1 – 2α) / (1 + 2α)] Pt (Pt is the total presure)
Partial pressure of nitrogen = Cα / C(1 + 2α) = [α / (1 + 2α)]Pt
Partial pressure of hydrogen = 3Cα / C(1 + 2α) = [3α / (1 + 2α)]Pt
Kp = [pN2][pH2]3 / [pNH3]2
Kp = [[pN2][pH2]3] / [pNH3]2
= [{α / [1 + 2α]} Pt {3α / [1 + 2α]}3] / [{1 − 2α / [1 + 2α]Pt}2]
= 27α4 Pt2 / [[1 + 2α]2[1 − 2α]2]
= 27α4 Pt2 / 1 – 4α2
Knowing Kp and the total pressure degree of dissociation of ammonia can be calculated.
2. From Vapour Density Measurements
- Vapour Density and Number of Moles
For ideal gases, pV = nRT = [ω / M] × RT
M = [ωRT] / VP = ρ [RT] / P = ρ[RTV] / RTn = ρ V / n = 2 × Vapour Density
Vapour density = ρ × V / 2n = α × 1 / n
At equilibrium V and ρ are constant and Vapour Density is α × 1 / n - Vapour Density and Equilibrium
Vapour density at start/ Vapour density at equilibrium = D / d = M / m = Moles at equilibrium / Moles at the start.
M = initial molecular weight and m = molecular weight at equilibrium
Example: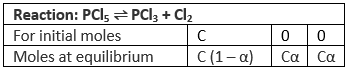 Number of moles at equilibrium = C (1 – α) + Cα + Cα = C (1 + α)
Number of moles at equilibrium = C (1 – α) + Cα + Cα = C (1 + α)
C (1 + α) C = D/d : 1 + α
α = D / d – 1
Knowing D and d, or M and m, α can be calculated.
Equilibrium Constant, Reaction Quotient, and Gibbs Free Energy
K is the ratio of the relative amount of products to reactants at equilibrium while Q is the ratio at any point of time of the reaction. The Q value can be compared to K to determine the direction of the reaction to take place. The spontaneity of the process is related to the free energy change.
△G (Gibbs Free Energy), K (Equilibrium Constant), and Q (Reaction Quotient) are related as follows:
- △G < 0 and Qc ˂ Kc or Kp at the start of the reaction: The reaction will proceed to form products.
- △G = 0 and Qc = Kc or Kp at equilibrium and no more changes in the concentration of the mixture.
- △G > 0 and Qc > Kc or Kp after equilibrium: The reaction will proceed in the direction to form reactants.
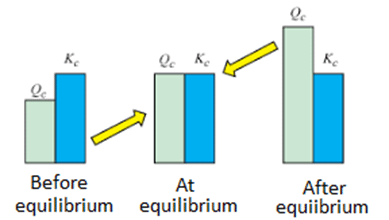 Equilibrium Constant vs Reaction Quotient
Equilibrium Constant vs Reaction Quotient
(i) Kc = Equilibrium constant measured in moles per liter.
(ii) Kp = Equilibrium constant calculated from the partial pressures
Relationship Between ΔGo & K
- Free energy, G, denotes the self-intrinsic electrostatic potential energy of a system. This means that in any molecule if we calculate the total electrostatic potential energy of all the charges due to all the other charges, we get what is called the free energy of the molecule. It tells about the stability of a molecule with respect to another molecule. The lesser the free energy of a molecule more stable it is.
- Every reaction proceeds with a decrease in free energy. The free energy change in a process is expressed by ΔG. If it is negative, it means that the product has lesser G than reactants, so the reaction goes forward. If it is positive the reaction goes reverse and if it is zero the reaction is at equilibrium.
- ΔG is the free energy change at any given concentration of reactants and products. If all the reactants and products are taken at a concentration of 1 mole per liter, the free energy change of the reaction is called ΔGo ( standard free energy change ).
- One must understand that ΔGo is not the free energy change at equilibrium. It is a free energy change when all the reactants and products are at a concentration of 1 mole/L. ΔGo is related to K (equilibrium constant ) by the relation, ΔGo= -RT ln K.
- K may either be Kc or Kp. Accordingly, we get
. The units of ΔGo depend only on R.
- T is always in Kelvin, and if R is in Joules, ΔGo will be in joules, and if R is calories then ΔGo will be in calories.
|
114 videos|263 docs|74 tests
|
FAQs on Equilibria: Types & Relation Between K, Q & G - Chemistry Class 11 - NEET
| 1. What is the difference between homogeneous and heterogeneous equilibria? |  |
| 2. How is the equilibrium constant related to the reaction quotient and Gibbs free energy? |  |
| 3. What are some applications of the equilibrium constant? |  |
| 4. How do you calculate the equilibrium concentration? |  |
| 5. What is the relationship between the equilibrium constant (K), reaction quotient (Q), and Gibbs free energy (G)? |  |
















