Essential Plant Nutrients | Agriculture Optional for UPSC PDF Download
The soil as a Nutrient Source for Plants
Mineral Nutrients in the Soil
Mineral nutrients in the soil exist in two forms: dissolved and bound. A very small fraction, less than 0.2%, of these nutrients are found in a dissolved state in soil water. The majority, nearly 98%, is either bound within organic detritus, humus, relatively insoluble inorganic compounds, or incorporated into minerals. These components make up a nutrient reserve, which is released slowly over time as a result of weathering and the breakdown of humus. The remaining 2% is attached to soil colloids. The soil solution, soil colloids, and the nutrient reserves in the soil are in a constant state of dynamic equilibrium, ensuring a continuous and gradual replenishment of nutrient elements.
Adsorption and Exchange of ions in the soil
Both clay minerals and humic colloids carry a negative electrical charge, which attracts and binds primarily cations (positively charged ions). There are also some positively charged sites where anions (negatively charged ions) can accumulate. The strength of the bond between a cation and these charged sites depends on the cation's charge and its level of hydration. Generally, ions with higher valences are held more tightly; for instance, Ca2+ is more strongly attracted than K+. Among ions with the same valence, those with less hydration are bound more firmly compared to those with strong hydration. The trend for cation adsorption follows the order Al3+, Ca2+, Mg2+, NH4+, K+, and Na+.
The presence of ions surrounding clay and humus particles acts as a mediator between the solid soil phase and the soil solution. If ions are introduced into or removed from the soil solution, an exchange occurs between the solid and liquid phases. This adsorptive binding of nutrient ions offers several advantages: it captures and safeguards nutrients released through weathering and humus decomposition, preventing them from being washed away; it helps maintain a low and relatively constant concentration in the soil solution, ensuring that plant roots and soil organisms are not exposed to extreme osmotic conditions. When needed by plants, these adsorbed nutrients are readily available.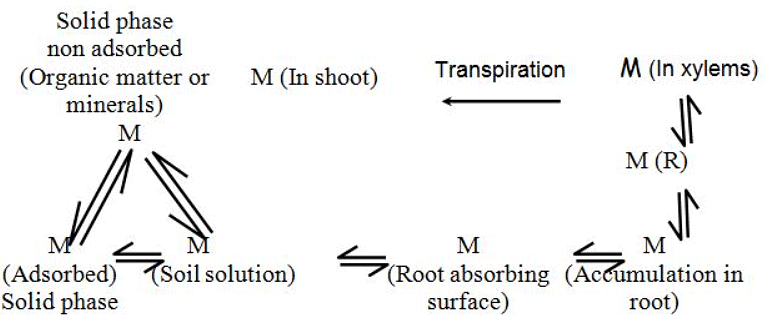 Nutrient release and path for absorption
Nutrient release and path for absorption
Essential and beneficial elements
The criteria of essentiality
To distinguish between essential elements for plant growth and those that can be taken up but are non-essential, Arnon (1954) established the following criteria:
- The plant must be incapable of normal growth or completing its life cycle in the absence of the element.
- The element is unique and cannot be substituted by another element.
- The element directly participates in metabolic processes.
Arnon's criteria for the essentiality of elements in plant growth are quite accurate, but from a practical perspective, they might be seen as too rigid. Nicholas introduced the term "Functional or Metabolic nutrients," regardless of whether their action is specific. Elements like sodium, cobalt, vanadium, and others are considered essential when a less strict definition of essentiality is applied.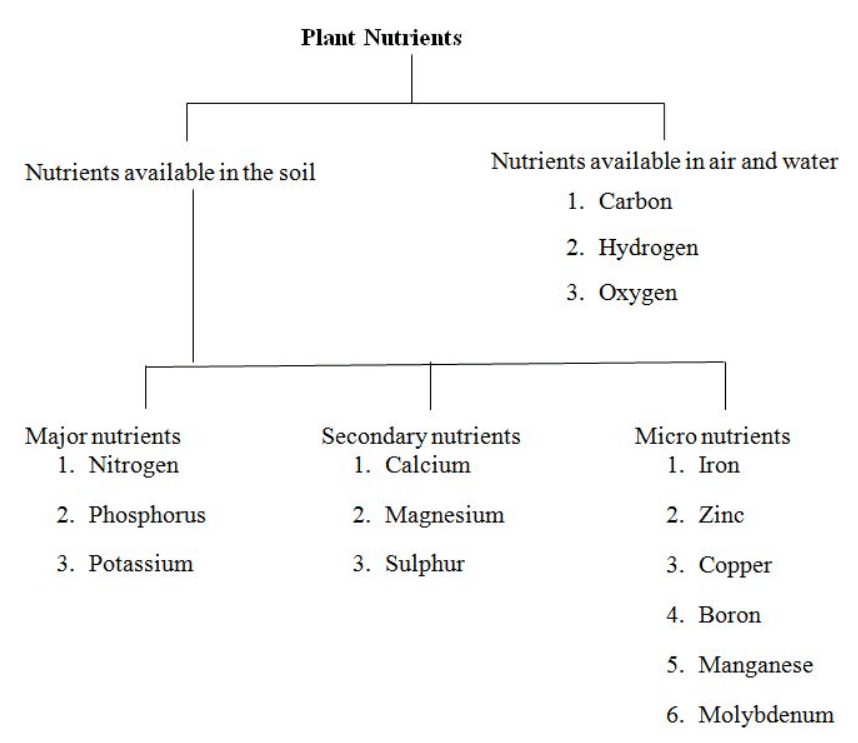
Classification of essential plant nutrients
(i) On the basis of amount of nutrients present in plants, they can be classified in to three groups:
Nutrients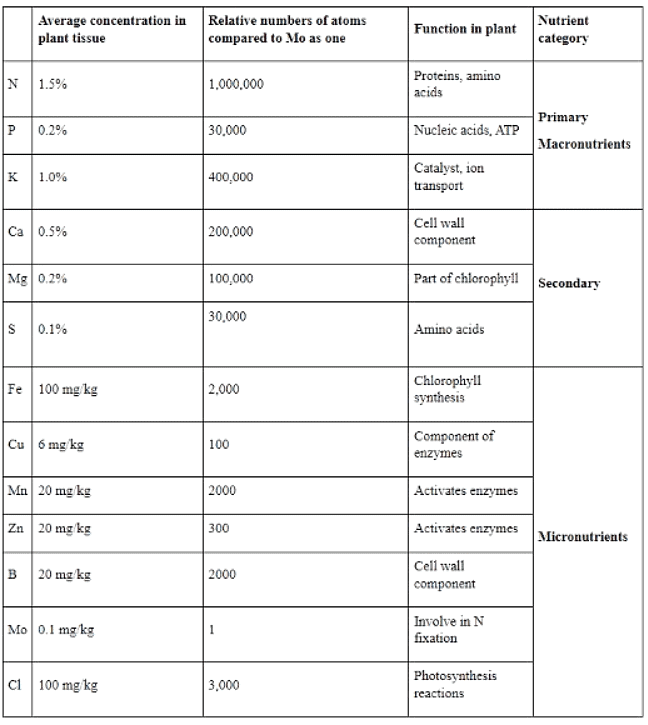
(ii) According to mobility
(a) In soil:
- Mobile: NO3-, SO42-, BO33-, Cl- and Mn2-
- Less mobile: NH42-, K+, Ca2+, Mg2+ and Cu2+
- Immobile: H2PO4-, HPO42- and Zn2+
(b) In plant:
- Highly mobile: N, P and K
- Moderately mobile: Zn
- Less mobile: S, Fe, Mn, Cu, Mo and Cl
- Immobile: Ca and B
(iii) According to metal and non metal
- Metal: K, Ca, Mg, Fe, Mn, Zn and Cu
- Non metal: N, P, S, B, Mo and Cl
(iv) According to cation and anion
- Cation: K, Ca, Mg, Fe, Mn, Zn and Cu
- Anion: NO3, H3PO4 and SO4
Beneficial elements
In addition to vanadium, silicon, aluminum, iodine, selenium, and gallium, which have been proven to be necessary for specific plant species, there are various other elements like rubidium, strontium, nickel, chromium, and arsenic. These elements, when present in very low amounts and often under specific conditions, have been observed to enhance the growth of particular plants or provide other advantageous effects. Although it hasn't been definitively established that these elements are essential for plant growth and metabolism, they are often referred to as "beneficial elements" due to their capacity to exert positive effects at extremely low concentrations.
Forms of nutrients in soil
In soil, Nutrient present in different forms are as under: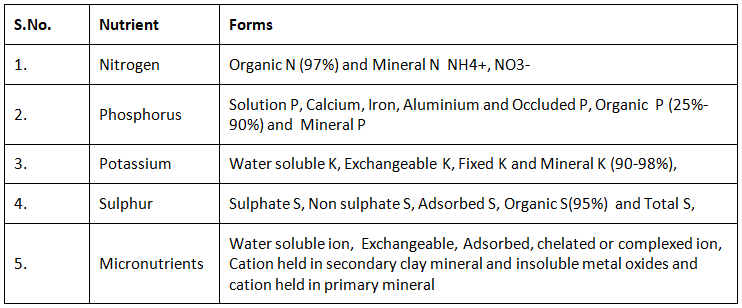
Mechanisms of nutrient transport to plants
Two important theories, namely, soil solution theory and contact exchange theory explain nutrient availability to plants.
Soil solution theory
- Mass flow: Movement of nutrient ions and salts along with moving water.
- Diffusion: Occurs when there is concentration gradient of nutrients between root surface and surrounding soil solution. Ions move from the region of high concentration to the region of low concentration.
Contact exchange theory
The important of contact exchange in nutrient transport is less than with soil solution movement. A close contact between root surface and soil colloids allows a direct exchange of ions.
Role of Macro and Micro-nutrients
Essential elements perform several functions. They participate in various metabolic processes in the plant cells such as permeability of cell membrane, maintenance of osmotic concentration of cell sap, electrontransport systems, buffering action, enzymatic activity and act as major constituents of macromolecules and co-enzymes.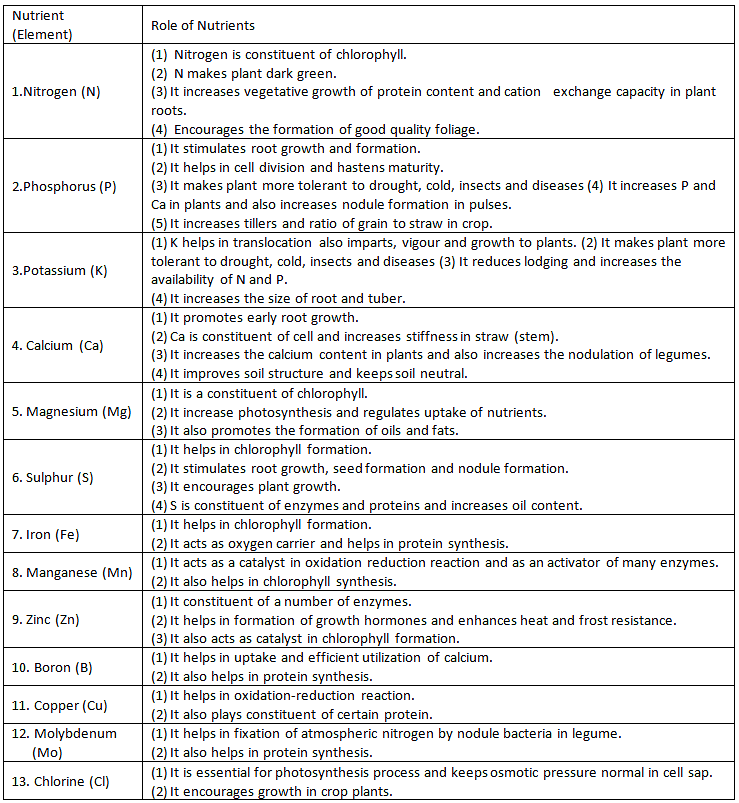
|
52 videos|224 docs
|
FAQs on Essential Plant Nutrients - Agriculture Optional for UPSC
| 1. What are the essential and beneficial elements for plants in soil? |  |
| 2. What are the different forms of nutrients in soil? |  |
| 3. How are nutrients transported to plants from the soil? |  |
| 4. What is the role of macro and micronutrients in plant nutrition? |  |
| 5. What are some frequently asked questions about the soil as a nutrient source for plants? |  |





















