F-Block Elements | Chemistry Class 12 - NEET PDF Download
| Table of contents |

|
| The f-block Elements (Inner Transition Elements) |

|
| Lanthanides |

|
| Physical and Chemical Properties of Lanthanides |

|
| Actinides |

|
| Physical and Chemical Properties of Actinides |

|
The f-block Elements (Inner Transition Elements)
The f-block encompasses two series: the lanthanoids, which comprise the fourteen elements succeeding lanthanum, and the actinoids, which consist of the fourteen elements succeeding actinium.
Lanthanides and Actinides are two series of elements that form the f-block of the periodic table. These elements are known for their unique electronic configurations, which involve filling the 4f and 5f orbitals, respectively.
- The lanthanoids exhibit a greater resemblance to each other compared to the members of typical transition elements within any series. They generally possess only one stable oxidation state, offering an excellent opportunity to investigate the impact of slight variations in size and nuclear charge along a series of otherwise similar elements.
- On the contrary, the chemistry of the actinoids is significantly more complex. This complexity arises from a broad spectrum of oxidation states in these elements and is further compounded by the challenges posed by their radioactivity, which introduces special complexities in their study.
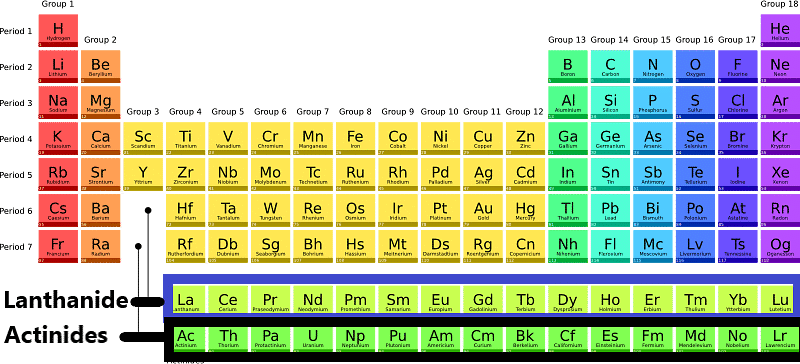 Lanthanide and ActinidesThe lanthanide series consists of 15 elements with atomic numbers ranging from 57 to 71, while the actinide series includes 15 elements with atomic numbers from 89 to 103.
Lanthanide and ActinidesThe lanthanide series consists of 15 elements with atomic numbers ranging from 57 to 71, while the actinide series includes 15 elements with atomic numbers from 89 to 103.
- Both series share common properties, and their study is crucial in understanding the behavior of elements under specific conditions.
- They are heavy metals and form colored ions. Lanthanides and Actinides are the elements that follow the rule of Afbau’s principle (It states that electrons get filled in lower-energy atomic orbitals before filling higher-energy ones).
They are placed under the f-block of the periodic table due to the preferential filling of 4f and 5f orbitals and due to this, the elements are kept separately in the modern periodic table.
Lanthanides
- The Lanthanide series includes 15 metallic chemical elements with atomic numbers 57 to 71, from Lanthanum through the Lutetium.
- The Lanthanide series derives its name from the first element in the series, Lanthanum.
- In Lanthanides, the electrons enter the penultimate 5d subshell and pre-penultimate 4f subshell.
- That is, Lanthanides is having an electronic configuration [Xe] 4fn+1 5d° 6s2 or [Xe] 4fn 5d1 6s2
- Here, [Xe] represents the electronic configuration of Radium which is the nearest noble gas.
- These elements, along with the chemically similar elements Scandium and Yttrium, are often collectively known as rare-earth elements or rare-earth metals.
Physical and Chemical Properties of Lanthanides
Below are the detailed properties of Lanthanides:
1. Electronic Configuration:
Lanthanides of the first f-block have a terminal electronic configuration of [Xe] 4f1-14 5d0-16s2 of the fourteen lanthanides.
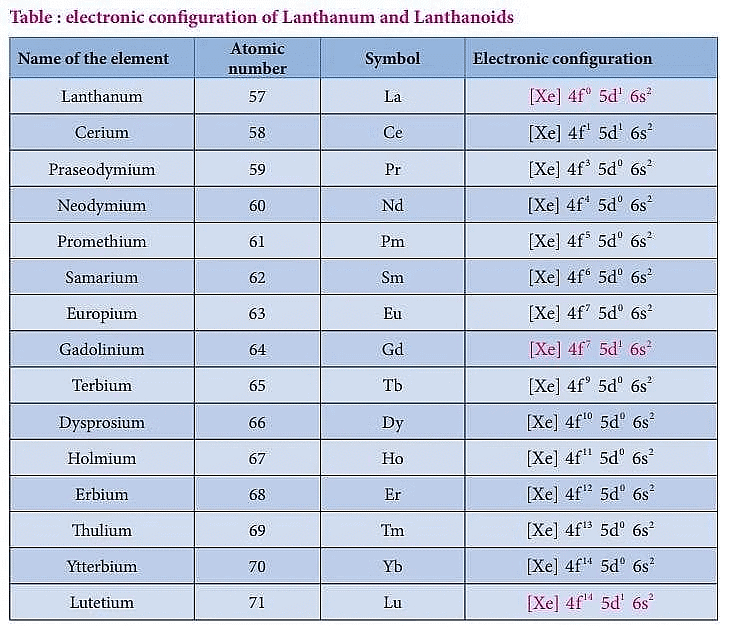
- Promethium (Pm), with atomic number 61, is the only synthetic radioactive element. The energy of 4f and 5d electrons are almost close to each other, so the 5d orbital remains vacant, and the electrons enter into the 4f orbital.
- Exceptions are in the case of gadolinium, Gd (Z = 64), where the electron enters the 5d orbital due to the presence of a half-filled d-orbital, and lutetium (Z = 71) enters the 5d orbital.
- Lanthanides consist of elements that follow lanthanum and involve the filling of 4f subshell
2. Lanthanide Contraction
The atomic size or the ionic radii of tri-positive lanthanide ions decrease steadily from La to Lu due to increasing nuclear charge and electrons entering the inner (n-2) f orbital. This gradual decrease in size with an increasing atomic number is called lanthanide contraction.
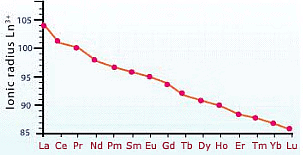 Lanthanide ContractionThe following points will clearly depict the effect of lanthanide contraction:
Lanthanide ContractionThe following points will clearly depict the effect of lanthanide contraction:
- Atomic size
- Difficulty in the separation of lanthanides
- Effect on the basic strength of hydroxides
- Complex formation
- The ionization energy of d-block elements
1. Similarity of 2nd and 3rd transition series i.e. 3d and 4 d series: The atomic sizes of second-row transition elements and third-row transition elements are almost similar. This is also an effect of lanthanide contraction. As we move down from form 4d to 5d series, the size must increase but it remains almost the same due to the fact that the 4f electrons present in the 5d elements show poor shielding effect.
2. Difficulty in the separation of lanthanides: Without lanthanide contraction all the lanthanides would have the same size because of which it would have been very difficult to separate them but due to lanthanide contraction their properties slightly vary. The variation in the properties is utilized for separating them.
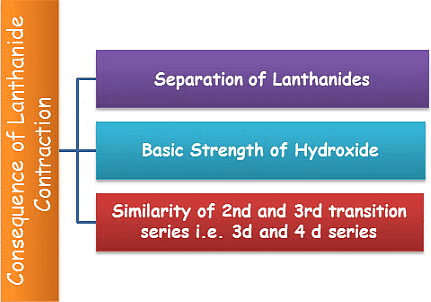
3. Effect on the basic strength of hydroxides: As the size of lanthanides decreases from La to Lu, the covalent character of the hydroxides increases, and hence their basic strength decreases. Thus, La (OH)3 is more basic, and Lu(OH)3 is the least basic.
4. Complex formation: Because of the smaller size but higher nuclear charge, the tendency to form coordinate complexes increases from La3+ to Lu3+. The lanthanides do not show much tendency to form complexes due to low charge density because of their size. However, the tendency to form complex and their stability increases with increasing atomic number.
5. Electronegativity: It increases from La to Lu.
6. Ionization energy: The attraction of electrons by the nuclear charge is much higher, and hence ionization energy of 5d elements is much larger than 4d and 3d. In the 5d series, all elements except Pt and Au have filled the s-shell. Elements from hafnium to rhenium have the same ionization energy, and after ionization energy increases with the number of shared d-electrons such that iridium and gold have the maximum ionization energy.
3. Oxidation States of Lanthanides
- Consistent +3 Oxidation State: All elements in the lanthanide series consistently exhibit an oxidation state of +3. Initial beliefs of possible +2 oxidation states for some metals (samarium, europium, and ytterbium) were clarified through subsequent studies, confirming a +2 oxidation state in solution complexes.
- Occasional +4 Oxidation States: Certain lanthanide metals occasionally show +4 oxidation states. This uneven distribution is attributed to the high stability of empty, half-filled, or fully-filled f-subshells.
- Stability of f-Subshell: The stability of the f-subshell significantly influences the oxidation state of lanthanides. Cerium favors a +4 oxidation state to achieve a noble gas configuration but tends to revert to +3, acting as a slow yet potent oxidant, even oxidizing water.
- +4 Oxidation State in Lanthanide Oxides: The +4 oxidation state is observed in the oxides of specific lanthanides, including Praseodymium (Pr), Neodymium (Nd), Terbium (Tb), and Dysprosium (Dy).
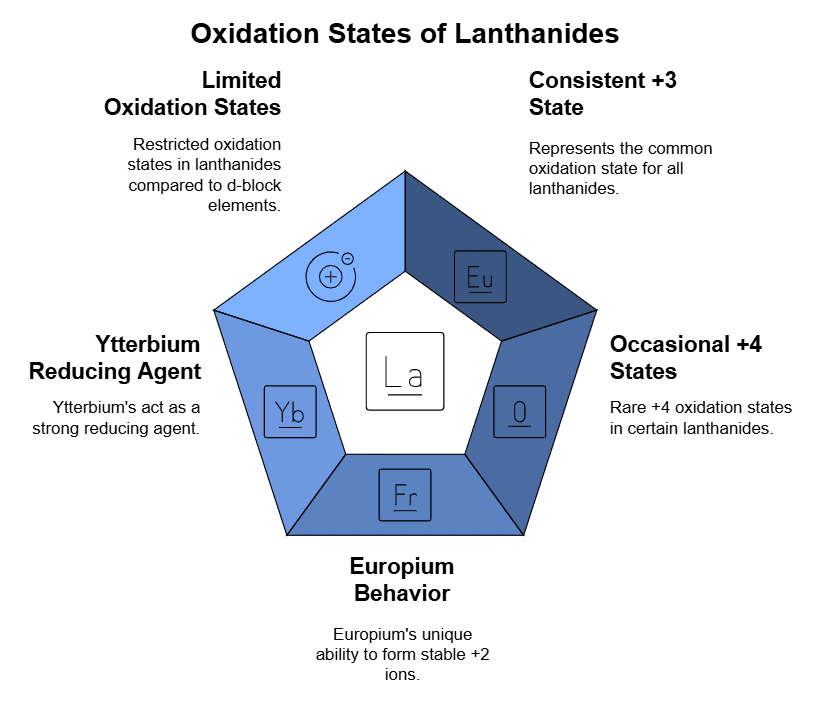
- Unique Behavior of Europium (Eu): Europium (atomic number 63) readily forms Eu2+ ions. Losing two electrons from the 6s energy level, it achieves a highly stable, half-filled 4f7 configuration. Transitions to the common oxidation state of lanthanides (+3) as Eu3+, acting as a robust reducing agent.
- Ytterbium (Yb) as a Strong Reducing Agent: Ytterbium (atomic number 70) exhibits similar characteristics as a strong reducing agent in the Yb2+ state. This behavior is attributed to its fully filled f-orbital.
- Influence of f-Subshell on Properties: The presence of the f-subshell has a profound influence on the oxidation state exhibited by lanthanides. Ongoing research and discoveries contribute to an evolving understanding of lanthanides.
- Limited Number of Oxidation States: Unlike d-block elements, lanthanides have a limited number of oxidation states. The substantial energy gap between 4f and 5d orbitals contributes to this limitation.
- Oxidation State in Aqueous Solution: In an aqueous solution, Sm2+, Eu2+, and Yb2+ lose electrons, i.e., get oxidized and are good reducing agents. On the other hand, Ce4+, Pr4+, and Tb4+ gain electrons, i.e., get reduced and are good oxidizing agents. Higher oxidation states (+4) of elements are possible only with oxides. For example, Pr, Nd, Tb, and Dy.
Question: What is the most common oxidation state of lanthanides?
The most common and stable oxidation state of lanthanides is +3. Some elements also exhibit +4 oxidation states. Some elements exhibit +2 oxidation state also due to their half-filled, fully-filled and noble gas configuration.
Question: Why Sm2+, Eu2+, and Yb2+ ions in solutions are good reducing agents but an aqueous solution of Ce4+ is a good oxidizing agent?
Solution:
+3 state is the most stable oxidation state of lanthanides. Thus the ions in +2 oxidation state tend to change to +3 oxidation state by loss of electron and those ions which are in +4 oxidation state tend to change to +3 oxidation state by gain of electron.
4. Chemical Reactivity of Lanthanides
All lanthanides share a common reactivity pattern, surpassing that of transition elements. This heightened reactivity arises from the shielding effect, where the outer 5s, 5p, and 5d orbitals protect the unpaired electrons in the inner 4f-orbital.
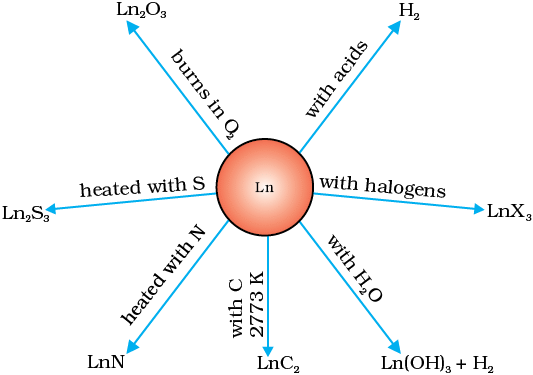 Oxidation and Tarnishing: Lanthanides readily tarnish upon exposure to oxygen. Oxides of the form M2O3 are formed, except for CeO2, which reacts with hydrogen to produce solid hydrides at temperatures between 300 and 400 degrees Celsius. Hydrides undergo decomposition in the presence of water.
Oxidation and Tarnishing: Lanthanides readily tarnish upon exposure to oxygen. Oxides of the form M2O3 are formed, except for CeO2, which reacts with hydrogen to produce solid hydrides at temperatures between 300 and 400 degrees Celsius. Hydrides undergo decomposition in the presence of water.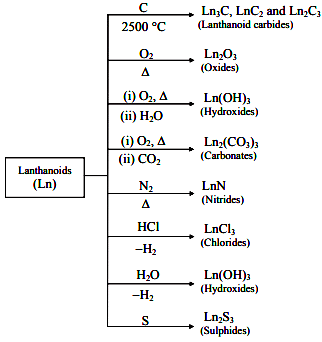 Halide Formation: Halides are synthesized by heating the metal with a halogen or reacting the oxide with ammonium halide. Chlorides exhibit deliquescence, while fluorides remain insoluble.
Halide Formation: Halides are synthesized by heating the metal with a halogen or reacting the oxide with ammonium halide. Chlorides exhibit deliquescence, while fluorides remain insoluble.- Solubility Characteristics: Nitrates, acetates, and sulfates of lanthanides display solubility. Carbonates, phosphates, chromates, and oxalates are insoluble in water.
- Reactivity Trends: Initial lanthanides exhibit reactivity similar to calcium, becoming more akin to aluminum with increasing atomic number.
- Chemical Combinations: Lanthanides combine with hydrogen upon gentle heating. Heating them with carbon results in the formation of carbides. Burning in the presence of halogens leads to the formation of halides.
- Acid Reactivity: Lanthanides react with dilute acids, liberating hydrogen gas.
- Oxides and Hydroxides: Lanthanides form oxides and hydroxides of the type N2O3 and M(OH)3, resembling basic alkaline earth metal oxides and hydroxides.
Actinides
The Actinide or Actinoid series includes 15 metallic chemical elements with atomic numbers from 89 to 103, actinium through Lawrencium.
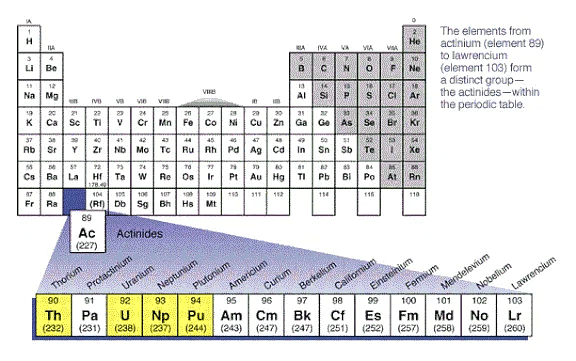 Position of Actinides in the Periodic Table
Position of Actinides in the Periodic Table
- The Actinide series derives its name from the first element in the series, Actinium.
- The Actinides have an electronic configuration
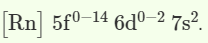
- Here, [Rn] represents the electronic configuration of Radium which is the nearest noble gas.
- The 6d and 5f subshell have a similar energy, so the electrons enter the 5f orbital.
- Actinides are highly electropositive and all elements are radioactive.
- Unlike the Lanthanides, most of the Actinide series elements have the same properties as the d
block.
Physical and Chemical Properties of Actinides
Below are the detailed properties of Actinides:
1. Electronic Configuration:
Actinides are the second series of elements of the f-block having a terminal electronic configuration of [Rn] 5f0-146d0-2 7s2 The energy of 5f and 6d electrons are close to each other, and so electrons enter into the 5f orbital.
2. Actinide Contraction
- The atomic radii of actinoids decrease steadily from Thorium to Lawrencium due to increasing nuclear charge.
- From the Thorium filling of 5f-subshell starts. So, due to the weak shielding effect of the f-subshell, the nuclear charge increases, and the contraction in the size of atoms takes place.
- The size contraction in actinoids is greater as compared to that in lanthanoids.
- Thus, due to the poor shielding effect of f- orbital actinoids undergo contractions called actinoids contraction.
3. Oxidation State of Actinides
Actinides show variable oxidation states because of the smaller energy gap between 5f, 6 d, and 7s orbitals.
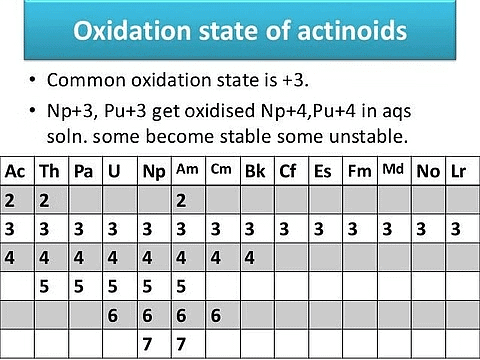 Oxidation of ActinoidsThough 3+ is the most stable oxidation state, other oxidation states are possible because of the good shielding of f-electrons.
Oxidation of ActinoidsThough 3+ is the most stable oxidation state, other oxidation states are possible because of the good shielding of f-electrons.
The maximum oxidation state first increases up to the middle of the series and then decreases, i.e. it increases from +4 for Th to +5, +6, and +7 for Pa, V, and Np, but decreases in the succeeding elements.
Question for F-Block ElementsTry yourself:Which is the most stable oxidation state of actinoids?View Solution
4. Formation of Complexes
Actinides are better complexing agents than lanthanides due to their smaller size but higher nuclear charge. They can form Pπ – complexes as well.
The degree of complexion decreases in the order M4+ > MO22+ > M3+ > MO22+.
5. Physical Properties of Actinides
- Density of Actinides: All actinides except thorium and americium have very high densities.
- Melting and Boiling Points of Actinides: Actinides have fairly high melting points, like lanthanides, but there is no definite trend in the melting and boiling points of lanthanides.
- Magnetic Properties of Actinides: All actinides are paramagnetic in nature, which depends on the presence of unpaired electrons. The orbital angular moment is quenched because of the shielding of 5f electrons so that the observed magnetic moment is less than the calculated.
- Ionization enthalpies: The actinides have lower ionization enthalpies as compared to lanthanides because 5f is more effectively shielded from nuclear charge than 4f.
Magnetic behavior: All actinides are paramagnetic in nature. The paramagnetic nature depends on the presence of unpaired electrons.
Radioactivity: All the actinides are radioactive in nature. Radioactivity increases with an increase in atomic number.
6. Chemical Reactivity of Actinides
- Electropositivity and Reactivity: Lower ionization energy makes actinides more electropositive than lanthanides. Higher reactivity was observed, reacting with hot water and oxidizing agents, resulting in the formation of a passive coating, along with the generation of halides and hydrides. Actinides function as strong reducing agents.
- Oxidation State Complexity: The capability to exist in various oxidation states adds complexity to actinides' chemistry. Radioactive nature poses challenges for laboratory studies of their chemical behavior.
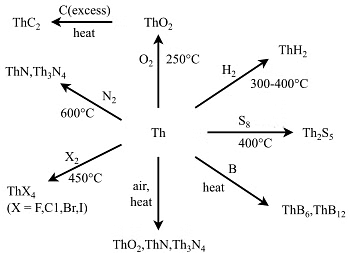 Chemical Reactivity of Thorium
Chemical Reactivity of Thorium
- Reaction with Boiling Water: React with boiling water, yielding a mixture of oxide and hydride.
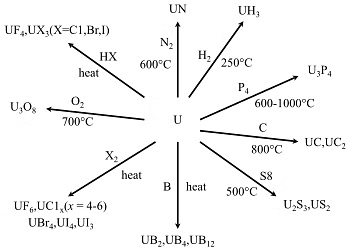
- Interaction with Non-metals: React with most non-metals at moderate temperatures.
- Acid Reactivity: Susceptible to attack by hydrochloric acid (HCl). Limited impact from nitric acid (HNO3) due to the formation of a protective oxide layer on their surface.
Question: Actinoids are mostly attacked by which acid?
Answer: Actinoids are highly reactive metals especially in the finely divided state. All these metals are attacked by hydrochloric acid but the effect of nitric acid is very small due to the formation of a protective oxide layer on their surface.
|
75 videos|339 docs|78 tests
|
FAQs on F-Block Elements - Chemistry Class 12 - NEET
| 1. What are the key physical properties of lanthanides? |  |
| 2. How do the chemical properties of lanthanides differ from those of actinides? |  |
| 3. What are some common applications of lanthanide elements? |  |
| 4. What are the main differences between lanthanides and actinides in terms of their occurrence and extraction? |  |
| 5. Why are actinides considered radioactive and what are the implications of their radioactivity? |  |





















