Class 10 Exam > Class 10 Notes > Physics for GCSE/IGCSE > Fission & Fusion
Fission & Fusion | Physics for GCSE/IGCSE - Class 10 PDF Download
Nuclear Fission
- The nucleus of an atom harbors significant energy, which can be liberated through processes like nuclear fission.
- Nuclear fission entails the division of a large, unsteady nucleus into two smaller nuclei.
- Both uranium and plutonium isotopes undergo fission and serve as fuel sources in nuclear power plants.
- In the course of fission, when a neutron interacts with an unstable nucleus, it prompts the nucleus to split into two smaller nuclei, termed daughter nuclei, along with the release of two or three neutrons.
- Additionally, gamma rays are emitted during this process.
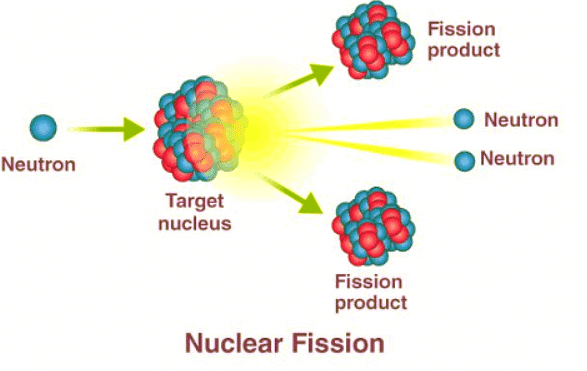
- Following fission, the resulting products disperse rapidly.
- Energy conversion occurs from nuclear potential energy to kinetic energy.
- The mass of the products, including daughter nuclei and neutrons, is less than that of the original nucleus.
- This reduction in mass signifies the conversion of the remaining mass into energy, which is discharged during the fission event.
- Various diagrams depict the mechanisms involved in nuclear fission, offering easily comprehensible illustrations of the reaction process.
- The diagram above is useful because it shows clearly the different parts of the fission reaction
- An example of a nuclide equation for fission is:
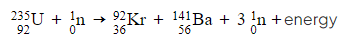
- Where:

- The equation above depicts a fission reaction where a Uranium nucleus is bombarded with a neutron, resulting in the division into two smaller nuclei – a Krypton nucleus and a Barium nucleus – while simultaneously emitting three neutrons.
- The sum of the top (nucleon) numbers on the left-hand side equates to the sum of the top numbers on the right-hand side: 235 + 1 = 92 + 141 + (3 × 1)
- Similarly, the lower (proton) numbers also adhere to this principle: 92 + 0 = 36 + 56 + (3 × 0)
Question for Fission & FusionTry yourself: What is the process of nuclear fission?View Solution
Nuclear Fusion
- Nuclear fusion involves the merging of two light nuclei to create a heavier nucleus.
- Sustaining extremely high temperatures is necessary for this process.
- The challenge of replicating nuclear fusion on Earth stems from this requirement.
- Stars harness nuclear fusion as an energy source.
- In many stars, hydrogen atoms undergo fusion to generate helium and release substantial energy.
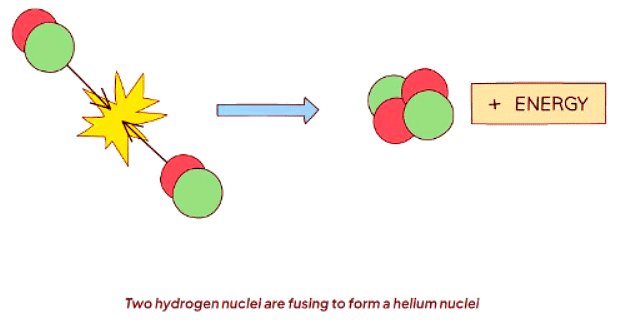
- The energy released in nuclear fusion originates from a tiny amount of the particle's mass converting into energy.
- Mass-Energy Equivalence: Described by Albert Einstein's famous equation.
- Einstein's famous equation, E = mc2
- Signifies the energy released from fusion:
- E = energy released from fusion in Joules (J)
- m = mass converted into energy in kilograms (kg)
- c = the speed of light in metres per second (m/s)
- Consequently, the mass of the resulting product (the fused nucleus) is less than the combined mass of the two initial nuclei.
- This reduction in mass occurs due to the conversion of the remaining mass into energy, which is liberated during the fusion process.
- The energy released during nuclear fusion is immense. For instance, the energy produced from 1 kg of hydrogen undergoing fusion is equivalent to burning approximately 10 million kilograms of coal.
- This fusion reaction is visually represented as:
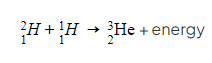
- Where:

The document Fission & Fusion | Physics for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Physics for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
126 videos|182 docs|35 tests
|
FAQs on Fission & Fusion - Physics for GCSE/IGCSE - Class 10
| 1. What is the difference between nuclear fission and nuclear fusion? |  |
Ans. Nuclear fission is the splitting of a heavy nucleus into two or more lighter nuclei, releasing energy, while nuclear fusion is the combining of two light nuclei to form a heavier nucleus, also releasing energy.
| 2. Which process, nuclear fission or fusion, is currently used in nuclear power plants? |  |
Ans. Nuclear fission is currently the process used in nuclear power plants to generate electricity.
| 3. What are the main advantages of nuclear fusion over nuclear fission? |  |
Ans. Nuclear fusion has the advantage of producing more energy per unit mass of fuel, creating less radioactive waste, and using abundant sources of fuel such as hydrogen isotopes.
| 4. Can nuclear fusion be achieved on Earth at the same scale as nuclear fission? |  |
Ans. While nuclear fusion has been achieved on Earth in experimental reactors, such as tokamaks, it has not yet been achieved at a commercial scale like nuclear fission power plants.
| 5. What are some of the challenges facing the development of nuclear fusion as a viable energy source? |  |
Ans. Challenges facing the development of nuclear fusion as a viable energy source include the high temperatures and pressures required for fusion reactions, containment of the plasma, and the development of materials that can withstand the harsh conditions inside a fusion reactor.
Related Searches




















