|
Reagents are substances that react with other substances to help identify or analyze them.
|
Card: 2 / 28 |
|
Fill in the blank: Sodium hydroxide and ammonium hydroxide are commonly used alkalis in analytical chemistry that react with ___ to help identify metal cations. |
Card: 3 / 28 |
|
Riddle: I am a solution that can identify metal ions, both strong and weak. What am I? |
Card: 5 / 28 |
|
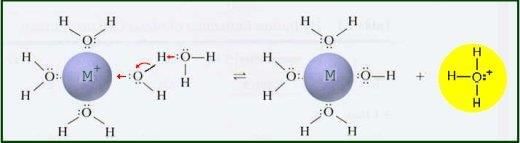
Alkalis like sodium hydroxide react with metallic salt solutions to form precipitates, which can indicate the presence of specific metal cations. |
Card: 8 / 28 |
|
Fill in the blank: Sodium hydroxide reacts with ___ to produce a soluble salt and water. |
Card: 9 / 28 |
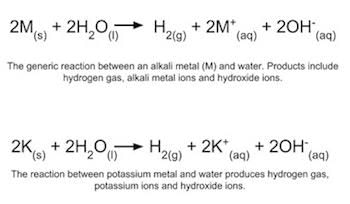 Alkalis can react with certain metals to produce hydrogen gas and a soluble metal hydroxide. |
Card: 12 / 28 |
|
Fill in the blank: The reaction between an alkali and a metal oxide often leads to the formation of ___ and water. |
Card: 13 / 28 |
 Unlock all Flashcards with EduRev Infinity Plan Starting from @ ₹99 only
|
|
Multiple Choice: What do alkalis typically produce when they react with amphoteric oxides? A) Hydrogen gas B) Soluble salts C) Insoluble compounds D) None of the above |
Card: 15 / 28 |
|
What is the primary difference between qualitative and quantitative analysis in analytical chemistry? |
Card: 17 / 28 |
|
Qualitative vs Quantitative Analysis
|
Card: 18 / 28 |
|
Fill in the blank: Sodium hydroxide and ammonium hydroxide are the most commonly used ___ in analytical chemistry. |
Card: 19 / 28 |
|
True or False: Ammonium hydroxide can be used to identify metal cations through characteristic tests. |
Card: 21 / 28 |
|
Riddle: I am used in labs, I can help you find, whether a substance is present or left behind. What am I? |
Card: 23 / 28 |
|
Fill in the blank: The characteristic tests of sodium hydroxide and ammonium hydroxide help in the identification of ___ . |
Card: 25 / 28 |
|
Alkalis are crucial laboratory reagents.
|
Card: 28 / 28 |