Important Electrochemistry Formulas for JEE and NEET
ELECTRODE POTENTIAL
For any electrode → oxidiation potential = - Reduction potential
Ecell = R.P of cathode - R.P of anode
Ecell = R.P. of cathode + O.P of anode
Ecell is always a +ve quantity & Anode will be electrode of low R.P
E°Cell = SRP of cathode - SRP of anode.
- Greater the SRP value greater will be oxidising power.
GIBBS FREE ENERGY CHANGE :
ΔG = - nFEcell
ΔG° = - nFE°cell
NERNST EQUATION : (Effect of concentration and temp of an emf of cell)
⇒ ΔG = ΔG° + RT lnQ (where Q is raection quotient)
ΔG° = - RT ln Keq

At chemical equilibrium
ΔG = 0 ; ECell = 0.
-


For an electrode M(s)/Mn+.
CONCENTRATION CELL : A cell in which both the electrods are made up of same material.
For all concentration cell E°cell = 0.
(a) Electrolyte Concentration Cell :
eg. Zn(s) / Zn2+ (c1) || Zn2+(c2) / Zn(s) 
(b) Electrode Concentration Cell:
eg. Pt, H2(P1 atm) / H+ (1M) / H2(P2atm) / Pt 
DIFFERENT TYPES OF ELECTRODES :
1. Metal-Metal ion Electrode M(s)/Mn+. Mn+ + ne- → M(s) 
2. Gas-ion Electrode Pt/H2(Patm)/H+(XM) as a reduction electrode 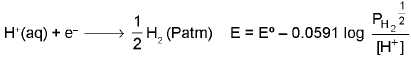
3. Oxidation-reduction Electrode Pt / Fe2+, Fe3+ as a reduction electrode Fe3+ + e- → Fe2+ 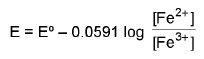
4. Metal-Metal insoluble salt Electrode eg. Ag/AgCI, Cl- as a reduction electrode AgCI(s) + e- → Ag(s) + Cl-
CALCULATION OF DIFFERENT THERMODYNAMICS FUNCTION OF CELL REACTION
- ΔG = -n FEcell
 (At costant pressure).
(At costant pressure).
 = Temperature cofficient of e.m.f of the cell.E = a + bT + CT2 + ....
= Temperature cofficient of e.m.f of the cell.E = a + bT + CT2 + ....
- ΔCp of cell reaction


ELECTROLYSIS :
(a) 
(b) Similarly the an ion which is strogner reducing agent(low value of SRP) is liberated fir stat the anode.
FARADAY’S LAW OF ELECTROLYSIS :
First Law :
Second Law :
W α E W/E = constant 

CURRENT EFFICIENCY = 
- CONDITION FOR SIMULTANEOUS DEPOSITION OF Cu & Fe AT CATHODE
 Condition for the simultaneous deposition of Cu & Fe on cathode.
Condition for the simultaneous deposition of Cu & Fe on cathode.
CONDUCTANCE:

- Specific conductance or conductivity :
(Reciprocal of specific resistance) k = 1/ρ K = specific conductance - Equivalent conductance:
 unit: -ohm-1 cm2 eq-1
unit: -ohm-1 cm2 eq-1 - Molar conductance:
 unit: -ohm-1 cm2 mole-1
unit: -ohm-1 cm2 mole-1
specific conductance = conductance x l/a
KOHLRAUSCH’S LAW:
Variation of λeq /λM of a solution with concentration :
(i) Strong electrolyte
(ii) Weak electrolytes : where λ is the molar conductivity
where λ is the molar conductivity
n+ = No of cations obtained after dissociation per formula unit
n_ = No of anions obtained after dissociation per formula unit
APPLICATION OF KOHLRAUSCH LAW:
1. Calculation of λ0M of weak electrolytes :
2. To calculate degree of diossociation of a week electrolyte
3. Solubility (S) of sparingly soluble salt & their Ksp

IONIC MOBILITY: It is the distance travelled by the ion per second under the potential gradient of 1 volts per cm. It’s unit is cm2s-1v-1.
Absolute ionic mobility:

Transport Number:
Where tc = Transport Number of cation & ta = Transport Number of anion
SOLUTION & COLLIGATIVE PROPERTIES
1. OSMOTIC PRESSURE :
(i) π = ρgh
Where, ρ = density of soln., h = equilibrium height.
(ii) Vont - Hoff Formula (For calculation of O.P.)
π = CST
π = CST = n/V (RT) (just like ideal gas equation)
∴ C = total cone, of all types of particles.
= C1 + C2 + C3 + ....
Note : If V1 mL of C1 cone. + V2 mL of C2 cone, are mixed. ;
; 
Type of solutions:
(a) Isotonic solution - Two solutions having same O.P.
π1 = π2 (at same temp.)
(b) Hyper tonic - If π1> π2. ⇒ Ist solution is hypertonic solution w.r.t. 2nd solution.
(c) Hypotonic - IInd solution is hypotonic w.r.t. Ist solution.
Abnormal Colligative Properties : (In case of association or dissociation)
VANT HOFF CORRECTION FACTOR (i):
- i > 1 ⇒ dissociation.
i < 1 ⇒ association. 
∴ π = iCRT
π = (i1C1 + i2C2 + i3C3.....) RT
Relation between i & α (degree of dissociation) :
i = 1 + (n - 1)α Where, n = x + y.
Relation b/w degree of association β & i.
2. RELATIVE LOWERING OF VAPOUR PRESSURE (RLVP) :
Vapour pressure : PSoln. < P
Lowering in VP = P - Ps = ΔP
Relative lowering in vapour pressure 
Raoult's law :- (For non - volatile solutes)
Experimentally relative lowering in V.P = mole fraction of the non volatile solute in solutions.

 (M = molar mass of solvent)
(M = molar mass of solvent)
If solute gets associated or dissociated
- According to Raoult’s law
(i) where X1 is the mole fraction o f the solvent (liquid).
where X1 is the mole fraction o f the solvent (liquid).
(ii) An alternate form →
- Ostwald—Walker Method : Experimental or lab determination of


3. ELEVATION IN BOILING POINT :
ΔTb = i x Kbm or
or 

4. DEPRESSION IN FREEZING POINT:
∴ ΔTf = i x Kf . m.
Kf = molal depression constant = 
RAOULT’S LAW FOR BINARY (IDEAL) MIXTURE OF VOLATILE LIQUIDS :
xA' = mole fraction of A in vapour about the liquid / solution.
xB' = mole fraction of B
Graphical Representation :

Ideal solutions (mixtures) : Mixtures which follow Raoul'ts law at all temperature.
ΔHmix = 0 ΔVmix = 0 ΔSmix = + ve as for process to proceed : ΔGmix = -ve
eg. (1) Benzene + Toluene.
(2) Hexane + heptane.
(3) C2H5Br + C2H5I.
Non - Ideal solutions : Which do not obey Raoult's law.
(a) Positive deviation :-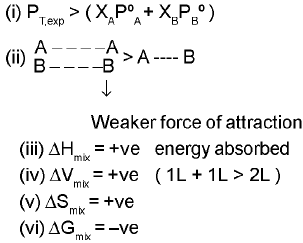
eg. H2O + CH3OH.
H2O + C2H5OH
C2H6OH + hexane
C2H5OH + cyclohexane.
CHCI3 + CCI4 → dipole dipole interaction becomes weak.
(b) Negative deviation
e.g. H2O + HCOOH
H2O + CH3COOH
H2O + HNO3
CHCl3 + CH3OCH3 ⇒ 

Immiscible Liquids:
(i) Ptotal= PA+ PB





B.P. of solution is less than the individual B.P.’s of both the liquids.
Henry Law :
This law deals with dissolution of gas in liquid i.e. mass of any gas dissolved in any solvent per unit volume is proportional to pressure of gas in equilibrium with liquid,
m α p
m = kρ
SOLID STATE
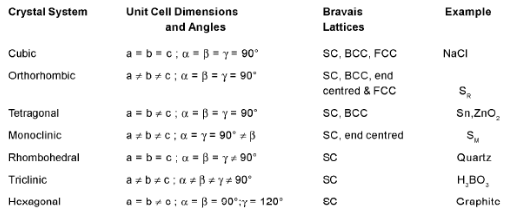
- Classification of Crystal into Seven System

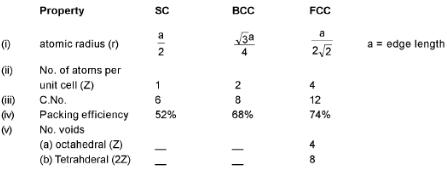
- ANALYSIS OF CUBICAL SYSTEM

- NEIGHBOUR HOOD OF A PARTICLE :
(i) Simple Cubic (SC) Structure :
(ii) Body Centered Cubic (BCC) Structure :
(iii) Face Centered Cubic (FCC) Structure:
- DENSITY OF LATTICE MATTER (d) =

where NA = Avogadro’s No. M = atomic mass or molecular mass. - IONIC CRYSTALS

- EXAMPLES OF A IONIC CRYSTAL
(a) Rock Salt (NaCI) Coordination number (6 : 6)
(b) CsCI C.No. (8 : 8) Edge length of unit cell:-
(c) Zinc Blende (ZnS) C.No. (4 : 4)
(d) Fluorite structure (CaF2) C.No. (8 : 4)
- Crystal Defects (Imperfections)

CHEMICAL KINETICS & REDIOACTIVITY
RATE/VELOCITY OF CHEMICAL REACTION : Mol lit-1 time-1 = mol dm-3 time-1
Mol lit-1 time-1 = mol dm-3 time-1
Types of Rates of chemical reaction :
For a reaction R → P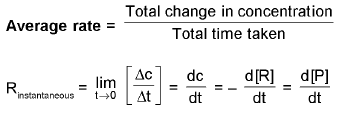

RATE LAW (DEPENDENCE OF RATE ON CONCENTRATION OF REACTANTS):
Rate = K (conc.)order - differential rate equation or rate expression
Where K = Rate constant = specific reaction rate = rate of reaction when concentration is unity unit of K = (conc)1-order time-1
Order of reaction :
m1A + m2B → products.
R ∝ [A]p [B]q Where p may or may not be equal to m1 & similarly q may or may not be equal to m2.
p is order of reaction with respect to reactant A and q is order of reaction with respect to reactant B and (p + q) is overall order of the reaction.
INTEGRATED RATE LAWS:
C0 or 'a' is initial concentration and Ct or a - x is concentration at time 't'
(a) zero order reactions :
Rate = k [cone.]0 = constant
Rate = k =  or Ct = C0 - kt
or Ct = C0 - kt
Unit of K = mol lit-1 sec-1, Time for completion = C0/k
 ∴ t1/2 ∝ C0
∴ t1/2 ∝ C0
(b) First Order Reactions :
(i) Let a 1st order reaction is, A → Products or
or 
⇒ Independent of initial concentration.
Independent of initial concentration.
Graphical Representation:


(c) Second order reaction :
2nd order Reactions Two types

(d) Psuedo first order reaction :
∴ For A + B → Products [Rate = K [A]1 [B]1]
Now if ‘B’ is taken in large excess b > > a.
⇒ 
∵ ‘b’ is very large can be taken as constant ⇒
⇒  k' is psuedo first order rate constant
k' is psuedo first order rate constant
METHODS TO DETERMINE ORDER OF A REACTION
(a) Initial rate method :
r = k[A]a [B]b [C]c if 
then for two different initial concentrations of Awe have ⇒
⇒ 
(b) Using integrated rate law : It is method of trial and error.
(c) Method of half lives :
for nth order reaction 
(d) Ostwald Isolation Method :
rate = k [A]a [B]b [C]c = k0 [A]a
METHODS TO MONITOR THE PROGRESS OF THE REACTION :
(a) Progress of gaseous reaction can be monitored by measuring total pressure at a fixed volume & temperature or by measuring total volume of mixture under constant pressure and temperature. 
 {Formula is not applicable when n = 1, the value of n can be fractional also.}
{Formula is not applicable when n = 1, the value of n can be fractional also.}
(b) By titration method:
1. ∴ a ∝ V0 a - x ∝ Vt ⇒ 
2. Study of acid hydrolysis of an easter.
(c) By measuring optical roteition produced by the reaction mixture:
EFFECT OF TEMPERATURE ON RATE OF REACTION. 2 to 3 (for most of the reactions)
2 to 3 (for most of the reactions)
Arhenius theroy of reaction rate.


Arhenius equation

If k1 and k2 be the rate constant of a reaction at two different temperature T1 and T2 respectively, then we have

 Ea ≥ 0
Ea ≥ 0
REVERSIBLE REACTIONS
In 


 ⇒
⇒ 
(ii) REVERSIBLE 1st ORDER REACATION ( both forward and backward)

(iii) SEQUENTIAL 1ST ORDER REACTION


CASE-I K1» K2 
CASE-II K2 » K1
|
108 videos|286 docs|123 tests
|

|
Explore Courses for NEET exam
|

|



 (At costant pressure).
(At costant pressure).
 = Temperature cofficient of e.m.f of the cell.E = a + bT + CT2 + ....
= Temperature cofficient of e.m.f of the cell.E = a + bT + CT2 + ....


 Condition for the simultaneous deposition of Cu & Fe on cathode.
Condition for the simultaneous deposition of Cu & Fe on cathode.
 unit: -ohm-1 cm2 eq-1
unit: -ohm-1 cm2 eq-1 unit: -ohm-1 cm2 mole-1
unit: -ohm-1 cm2 mole-1
 where X1 is the mole fraction o f the solvent (liquid).
where X1 is the mole fraction o f the solvent (liquid).