Fragmentations | Chemistry Optional Notes for UPSC PDF Download
| Table of contents |

|
| Grob Fragmentation |

|
| Solved Examples |

|
| 1,5-Fragmentations |

|
| Eschenmoser Fragmentation |

|
Grob Fragmentation
- The Grob fragmentation is similar to the pinacol rearrangement. Substrates that are 1,2-diols (or 1,2-diol type compounds) undergo the pinacol rearrangement to yield a new ketone or aldehyde. Substrates that are 1,3-diols (or 1,3-diol type compounds) undergo the Grob fragmentation to yield both a new ketone/aldehyde and an alkene, as shown below.

- The Grob fragmentation is an excellent method for constructing medium or large rings from bicyclic systems. Two examples for the synthesis of 10-membered rings are shown below. The example on the left is a standard Grob fragmentation. A deprotonated alcohol forms a carbonyl that promotes cleavage of the adjacent C-C bond (the fragmentation) to generate a new alkene and loss of the tosylate leaving group. The example on the right highlights that nitrogen can also function as a fragmentation promoting atom. In this case, fragmentation and loss of tosylate yields a new alkene and an iminium ion.
- Sodium borohydride reduces the iminium ion to yield the amine product. The example on the left is actually an oversimplification of this reaction because it does not show stereochemistry. Fragmentations are similar to E2 reactions and cyclic pinacol rearrangements in that the reacting groups must be antiperiplanar for constructive orbital overlap to occur. The follow problem explores the importance of stereochemistry for the Grob fragmentation.

Solved Examples
Example 1: Only one of the following two reactions yields a Grob fragmentation. Which reaction is it? Explain. Hint: It is very helpful to draw out these trans decalin molecules in the chair conformation.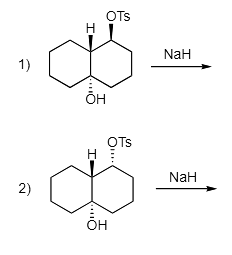 Ans: Reaction #1 undergoes fragmentation while reaction #2 does not. Focusing on the chair conformation for each molecule, we can explain why. In the first reaction, the fragmenting C-C bond and the bond to the leaving group are anti (both in red). In reaction #2, the fragmenting C-C bond and the leaving group (both in red) are not anti. Instead, a C-H bond is anti to the fragmenting C-C bond so the reaction does not occur.
Ans: Reaction #1 undergoes fragmentation while reaction #2 does not. Focusing on the chair conformation for each molecule, we can explain why. In the first reaction, the fragmenting C-C bond and the bond to the leaving group are anti (both in red). In reaction #2, the fragmenting C-C bond and the leaving group (both in red) are not anti. Instead, a C-H bond is anti to the fragmenting C-C bond so the reaction does not occur.

1,5-Fragmentations
Though much less common than Grob fragmentations, 1,5-fragmentations are also possible. These substrates start as ketones or aldehydes and the reaction is promoted by base induced enolate formation. Upon fragmentation, they yield an unsaturated ketone and an alkene, as shown below.

Example 2: Predict the product of the following reaction.

Ans: After the formation of the enolate, the molecule can fragment as shown above to yield the 11-membered ring product.

Eschenmoser Fragmentation
- The Eschenomser fragmentation (sometimes called the Eschenmoser-Tanabe fragmentation) was discovered in 1967 in the lab of Albert Eschenmoser at the Swiss Federal Institute of Technology (ETH) in Zurich, Switzerland. Much of the early work on the scope of this reaction in the Eschenmoser lab was conducted by Dorothee Felix. The reaction begins with the epoxidation of a cyclic conjugated enone to yield an epoxy carbonyl. Treatment of this molecule with tosyl hydrazine promotes fragmentation to remove the ring, generating a new carbonyl and alkyne.

- The mechanism of the fragmentation reaction is similar to the Wolff-Kishner reaction (reduction reaction of carbonyls with hydrazine and base) with important differences due to the presence of the epoxide and the tosylate group. After formation of the tosyl hydrazone, the important mechanistic steps begin. Movement of electrons from the nitrogen attached to the tosyl forms a double bond between the nitrogens, creates a C5-C6 alkene, and opens the epoxide. Next, the electrons flow back in the opposite direction. A carbonyl forms which cleaves the C2-C6 bond (the fragmentation) to yield the product alkyne while generating nitrogen gas (N2) and the tosyl leaving group.

Example 3: What is the product of the following reaction sequence?
 Ans: Like all Eschenmoser fragmentations, the reaction will ultimately cleave the alkene bond in the original conjugated enone. The means that the alkene carbon farthest from the carbonyl (the beta carbon) will turn into the product ketone while the other carbon from the alkene and the ketone carbon will turn into the product alkyne. Applying this along with the mechanism show above yields the target, containing one fewer ring than the starting material.
Ans: Like all Eschenmoser fragmentations, the reaction will ultimately cleave the alkene bond in the original conjugated enone. The means that the alkene carbon farthest from the carbonyl (the beta carbon) will turn into the product ketone while the other carbon from the alkene and the ketone carbon will turn into the product alkyne. Applying this along with the mechanism show above yields the target, containing one fewer ring than the starting material.

FAQs on Fragmentations - Chemistry Optional Notes for UPSC
| 1. What is grob fragmentation? |  |
| 2. What is the Eschenmoser fragmentation? |  |
| 3. How many types of fragmentations are there in organic chemistry? |  |
| 4. What is the significance of fragmentations in organic chemistry? |  |
| 5. How are fragmentations relevant to the UPSC exam? |  |

|
Explore Courses for UPSC exam
|

|
















