Configuration & Properties of d-Block Elements | Chemistry Class 12 - NEET PDF Download
What are the d-block Elements?
The elements present in the middle of the periodic table from Groups 3 to 12 are called d-block elements. The name d- block is derived from the fact that the last electron enters into the d-orbital of the penultimate shell.

Transition elements: These are often called transition elements because their characters lie between highly reactive metallic elements of s-block and nonmetallic elements of p-block elements. There are four series in the d block corresponding to the filling up of 3d, 4d, 5d or 6d orbitals.
- First transition series or 3d series ( Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn)
- Second transition series or 4d series (Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd)
- Third transition series or 5d series (La, Hf, Ta, W, Re, Os, Ir, Pt, Au, Hg)
- Fourth transition series or 6d series- incomplete.

Some Important Points
- Partially filled d orbitals: Transition elements have d subshells that are only partially occupied by electrons. This electron configuration distinguishes them from other elements.
 Position on the periodic table: Generally, transition elements are found in the d-block of the modern periodic table, specifically in groups 3 to 12. These groups include elements like titanium, iron, and copper.
Position on the periodic table: Generally, transition elements are found in the d-block of the modern periodic table, specifically in groups 3 to 12. These groups include elements like titanium, iron, and copper. - Exceptions: Not all elements in the d-block are classified as transition elements. Mercury, cadmium, and zinc, for instance, do not meet the criteria due to their electronic configurations, which match the (n-1)d10 ns2 pattern.
- Ground state and oxidation states: Transition elements can have fully filled d orbitals in their ground states or specific oxidation states. For instance, mercury can exhibit the +2 oxidation state with an electron configuration of (n-1)d10.Question for Configuration & Properties of d-Block ElementsTry yourself:Which of the following best describes transition elements according to the IUPAC definition?View Solution
Electronic Configuration of d- block Elements
The external electronic configuration is constant, with a gradual filling of 3d orbitals across the series starting from scandium.
- However, the filling is not regular, as chromium and copper have an increase in the 3d orbitals by gaining an electron from the 4s shell.
- In chromium, both the 3d and 4s orbitals are occupied, but neither is completely filled. This shows that the energies of the 3d and 4s orbitals are quite close for elements in this row.
The electronic configurations of the first, second, and third series elements are as follows:
- First transition series or 3d series ( Sc, Ti, V, Cr, Mn, Fe, Co, Ni, Cu, Zn)
1s2 2s2 2p6 3s2 3p6 3 d1-10 4s1-2 - Second transition series or 4d series (Y, Zr, Nb, Mo, Tc, Ru, Rh, Pd, Ag, Cd)
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1-2 4d1-10 - Third transition series or 5d series (La, Hf, Ta, W, Re, Os, Ir, Pt, Au, Hg)
1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 4f0-14 5d1-106s2 - Fourth transition series or 6d series- incomplete.
- These three series of elements depend on the (n-1) d orbital that is being filled. An orbital of lower energy is filled first.
- Therefore, 4s orbital with lesser energy is filled first to its full capacity. After 4s, the 3d orbital with higher energy is filled.
- The precisely, half-filled and fully filled d-orbitals are exceptionally stable.

The table below shows the first two rows of transition elements and their respective electronic configurations. It is worth noting that some of these elements have an electron configuration of (n-1)d5 ns1 or (n-1)d10 ns1. This is due to the stability offered by half-filled or fully-filled electron orbitals.

It has been noticed that many transition elements, such as chromium, do not follow the Aufbau principle. This is thought to be due to the small energy difference between the 3d and 4s orbitals, as well as the 4d and 5s orbitals.
Properties of Transition Elements (d Block)
Transition elements, also known as transition metals, exhibit several distinctive properties due to their partially filled d orbitals and position in the periodic table.
Some of the key properties of transition elements are as follows:
1. Physical Properties
(i) Metallic Characteristics:
- Most transition elements exhibit typical metallic characteristics like high tensile strength, ductility, malleability, elevated thermal and electrical conductivity, and a metallic luster.
- Except for zinc (Zn), cadmium (Cd), mercury (Hg), and manganese (Mn), they generally possess one or more common metallic structures under normal temperatures.
(ii) Hardness and Volatility:
- Transition metals, excluding Zn, Cd, and Hg, are characterized by their high hardness and low volatility.
(iii) Melting and Boiling Points:
- The transition metals, specifically those in the 3d, 4d, and 5d series, exhibit elevated melting and boiling points.
- The high melting points of these metals result from the significant contribution of electrons from the (n-1)d orbitals, in addition to the ns electrons, in interatomic metallic bonding.
- Within each row, the melting points of these metals reach a peak at d5, except for irregular values observed in the cases of Mn and Tc, and then consistently decrease with the increasing atomic number.
(iv) Enthalpy of Atomization:
- The transition metals exhibit elevated enthalpies of atomization. The peaks around the midpoint of each series suggest that having one unpaired electron per d orbital is particularly advantageous for establishing strong interatomic interactions. In general, a higher number of valence electrons contributes to stronger bonding.
- The enthalpy of atomization plays a crucial role in determining the standard electrode potential of a metal, and metals with exceptionally high enthalpies (indicating very high boiling points) tend to exhibit noble behavior in their reactions, as discussed later in the context of electrode potentials.

- Furthermore, a noteworthy observation is that metals in the second and third series display greater enthalpies of atomization compared to their counterparts in the first series.
- This observation is significant in explaining the more frequent occurrence of metal–metal bonding in compounds of heavy transition metals.
(v) Density:
Transition elements typically have high densities, which can be attributed to their compact atomic structures and the presence of heavy elements in the d-block of the periodic table.
Other Properties-
2. Variation in Atomic and Ionic Radii
The atomic and ionic radii of the transition elements decrease from group 3 to group 6 due to the poor shielding offered by the small number of d-electrons. Those placed between groups 7 and 10 have somewhat similar atomic radii and those placed in groups 11 and 12 have larger radii. This is because the nuclear charge is balanced out by the electron-electron repulsions.
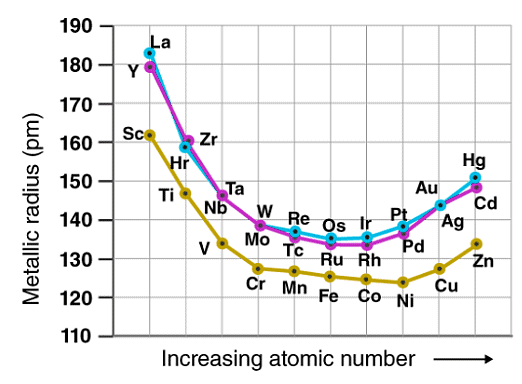
While traversing down the group, an increase in the atomic and ionic radii of the elements can be observed. This increase in the radius can be explained by the presence of a greater number of subshells.
3. Ionization Enthalpy
- The ionization enthalpies of d-block elements increase progressively due to changes in their atomic structure over time. The initial three ionization enthalpies of the first-row d-block elements are detailed in the adjacent table.
- Considering that the removal of one electron influences the relative energies of the 4s and 3d orbitals, we can elucidate the erratic trends observed in the first ionization enthalpy of transition metals. Consequently, unipositive ions typically result in dn configurations without 4s electrons, especially unipositive ions. The transfer of s electrons into d orbitals during ionization introduces reorganization energy, along with some gains in exchange energy, with the increasing number of electrons.

- The first ionization enthalpies of transition elements follow a typical trend of increasing from left to right across the periodic table. In the case of chromium (24), the change in initial ionization enthalpy is relatively minor since there is no alteration in the d orbital configuration. Conversely, zinc (30) experiences a more substantial change because its ionization involves the removal of an electron from the 4s orbital configuration.
- For chromium (Cr) and copper (Cu), the second ionization enthalpy is relatively high due to disruptions in the d5 and d10 configurations of M+ ions. In contrast, for zinc (Zn), the value is comparatively low because the ionization entails the removal of an electron, resulting in the formation of the stable d10 configuration.
- The increase in the third ionization enthalpy, not involving 4s orbitals, makes the removal of an electron from Mn2+ (d5) and Zn2+ (d10) ions more challenging than it would be otherwise. Consequently, there is a notable difference between the third ionization enthalpies of iron (26) and manganese (25).
4. Oxidation states
- Transition elements, especially those located in or near the middle of the series, exhibit a wide range of oxidation states. Manganese, for instance, can display oxidation states ranging from +2 to +7. The limited number of oxidation states at the extremes of the series results from either a scarcity of electrons available for sharing or losing (as seen in Sc and Ti) or an excess of d electrons, leading to fewer orbitals available for higher valence (as observed in Cu and Zn).
- Consequently, early in the series, scandium(II) is rarely encountered, and titanium (IV) is more stable than Ti(III) or Ti(II). Conversely, at the other end, zinc only assumes an oxidation state of +2, with no involvement of d electrons.

- The maximum oxidation states of reasonable stability align with the sum of the s and d electrons up to manganese (TiIVO2, VVO2+, MnVIIO4–). However, there is a marked decline in the stability of higher oxidation states beyond manganese, leading to the typical oxidation states observed in species like FeII, III, CoII, III, NiII, CuI, II, and ZnII.
- The variability in oxidation states, a distinctive trait of transition elements, arises due to the incomplete filling of d orbitals, resulting in oxidation states differing by unity (e.g., VII, VIII, VIV, VV), in contrast to non-transition elements where oxidation states typically differ by two units.
- An interesting observation in the variability of oxidation states among the d-block elements occurs within groups 4 through 10. Unlike the p-block where lower oxidation states are favored by heavier members due to the inert pair effect, the opposite is true in the d-block groups. For instance, in group 6, Mo(VI) and W(VI) are more stable than Cr(VI). Consequently, Cr(VI) in the form of dichromate in an acidic medium acts as a strong oxidizing agent, whereas MoO3 and WO3 do not act as strong oxidizing agents.
- Low oxidation states are prevalent when complex compounds have ligands with π-acceptor character and σ-bonding. Examples include Ni(CO)4 and Fe(CO)5, where the oxidation state of nickel and iron is zero.
5. Trends in the M 2+/M Standard Electrode Potentials
- Cu exhibits a distinctive behavior with a positive E-, which explains its inability to release H2 from acids. Cu reacts only with oxidizing acids such as nitric and hot concentrated sulfuric acids, where the acids undergo reduction. Its hydration enthalpy does not offset the high energy required to transform Cu(s) to Cu2+(aq).

- The overall trend towards less negative E- values across the series is associated with the general increase in the sum of the first and second ionization enthalpies. Notably, the E- values for Mn, Ni, and Zn are more negative than expected from the trend, providing an interesting observation.
- The E- values for Mn2+ and Zn2+ are influenced by the stability of the half-filled d sub-shell in Mn2+ and the filled d10 configuration in Zn2+. In contrast, for Ni, the E- value is associated with the most negative ∆hydH-.
Observed and calculated values for the standard electrode potentials (M2+ → M°) of the elements Ti to Zn
6. Trends in the M3+/M2+ Standard Electrode Potentials
- The low E- value for Sc can be attributed to the stability of Sc3+, which possesses a noble gas configuration.
- On the other hand, the highest E- value for Zn is a result of electron removal from the stable d10 configuration of Zn2+.
- Mn exhibits a relatively high value, indicating the pronounced stability of Mn2+ (d5), whereas the comparatively low value for Fe underscores the enhanced stability of Fe3+ (d5). The relatively low E- value for V is linked to the stability of V2+.
7. Trends in Stability of Higher Oxidation States
- Chromium, manganese, and cobalt are capable of attaining higher oxidation states. In the context of halides, manganese typically doesn't display a +7 oxidation state, but the compound MnO3F is an exception. In the case of copper, Cu+2(aq) is more stable than Cu+(aq) due to the higher ΔhydH of Cu+2, which compensates for the second ionization enthalpy of copper.
- Manganese exhibits elevated oxidation states in oxides; for instance, in Mn2O7, the oxidation state of Mn is +7. While manganese fluorides like MnF4 don't showcase such high oxidation states, manganese oxides, such as Mn2O7, demonstrate them. In Mn2O7, each manganese atom is tetrahedrally surrounded by oxygen atoms, including a Mn-O-Mn bridge. Conversely, other elements in the same group, such as iron with Fe2O3 exhibiting a +3 oxidation state and vanadium with V2O4 in a +4 oxidation state, present different oxidation states.
- In the p-block elements, a tendency is observed where lower oxidation states are favored by heavier members due to the inert pair effect. However, in the d-block, an opposite trend is noted, such as in group 6 where molybdenum (Mo) in the +6 oxidation state is found to be more stable compared to chromium (Cr) in the same oxidation state.
8. Chemical Reactivity and E- Values
- Transition metals exhibit diverse chemical reactivity, with many being electropositive enough to dissolve in mineral acids. However, some are considered 'noble metals' as they remain unaffected by individual acids. In the first series of metals, except for copper, they are relatively more reactive and can be oxidized by 1M H+, although the reaction rates with oxidizing agents like hydrogen ions (H+) can be slow. For instance, titanium and vanadium may be passive to dilute non-oxidizing acids at room temperature.
- The E- values for M2+/M suggest a decreasing tendency to form divalent cations across the series. This trend towards less negative E- values is correlated with the increase in the sum of the first and second ionization enthalpies. Notably, the E- values for Mn, Ni, and Zn are more negative than expected from the general trend. The stability of the half-filled d subshell (d5) in Mn2+ and the filled d subshell (d10) in zinc is associated with their Ee values. For nickel, the Eo value is linked to the most negative enthalpy of hydration.
- Mn3+ and Co3+ ions are particularly strong oxidizing agents in aqueous solutions. Conversely, Ti2+, V2+, and Cr2+ ions are potent reducing agents capable of liberating hydrogen from a dilute acid.
9. Magnetic Properties
- When a substance is subjected to a magnetic field, it exhibits two main types of magnetic behavior: diamagnetism and paramagnetism.
- Diamagnetic substances are repelled by the applied magnetic field, while paramagnetic substances are attracted. If a substance is attracted very strongly, it is considered ferromagnetic, with ferromagnetism being an extreme form of paramagnetism. Many transition metal ions display paramagnetic behavior.
- Paramagnetism arises from the presence of unpaired electrons, each carrying a magnetic moment associated with its spin angular momentum and orbital angular momentum.

- For compounds of the first series of transition metals, the contribution of orbital angular momentum is effectively suppressed and is thus negligible. In these cases, the magnetic moment is determined by the number of unpaired electrons, calculated using the 'spin-only' formula: µ = root[n(n+2)], where n represents the number of unpaired electrons, and µ is the magnetic moment in units of Bohr magneton (BM). A single unpaired electron has a magnetic moment of 1.73 Bohr magnetons.
- The magnetic moment increases with the growing number of unpaired electrons. Therefore, the observed magnetic moment serves as a useful indication of the number of unpaired electrons present in the atom, molecule, or ion.
Calculated and Observed Magnetic Moments (BM)
10. Formation of colored compounds
Transition metal compounds often exhibit vibrant colors. This is due to the presence of partially filled d orbitals that can absorb certain wavelengths of light, resulting in the observed color. Coloured Complex Formation
Coloured Complex Formation
11. Formation of Complex Compounds
- Complex compounds are characterized by metal ions binding with several anions or neutral molecules, resulting in the formation of complex species with distinctive properties.
- Examples of such complexes include [Fe(CN)6]3–, [Fe(CN)6]4–, [Cu(NH3)4]2+, and [PtCl4]2–.
- Transition metals, owing to their smaller sizes, higher ionic charges, and the presence of d orbitals available for bonding, are capable of forming a significant number of complex compounds.
12. Catalytic Properties
- The catalytic activity of transition metals and their compounds is well-known, attributed to their capability to assume various oxidation states and form complexes.
- Examples include vanadium(V) oxide in the Contact Process, finely divided iron in Haber Process, and nickel in Catalytic Hydrogenation.
- Catalysis at a solid surface involves the creation of bonds between reactant molecules and atoms on the catalyst's surface, with first-row transition metals utilizing 3d and 4s electrons for bonding.
- This leads to an increase in the concentration of reactants at the catalyst surface and a weakening of bonds in the reacting molecules, ultimately lowering the activation energy.
- Additionally, the ability of transition metal ions to change their oxidation states enhances their effectiveness as catalysts.
13. Formation of Interstitial Compounds
- Interstitial compounds are created when small atoms like H, C, or N are enclosed within the crystal lattices of metals. These compounds are typically non-stoichiometric and don't conform to the typical characteristics of ionic or covalent compounds.
- Examples include TiC, Mn4N, Fe3H, VH0.56, and TiH1.7. The formulas provided don't align with the usual oxidation states of the metals involved. Due to their unique composition, these compounds are termed interstitial compounds.
- Key physical and chemical traits of interstitial compounds include:
(i) They possess high melting points, exceeding those of pure metals.
(ii) They exhibit exceptional hardness, with some borides approaching the hardness of diamonds.
(iii) They maintain metallic conductivity.
(iv) They are chemically inert.
14. Alloy Formation
- An alloy is a combination of metals created by mixing their components. These alloys can be homogeneous solid solutions, where the atoms of one metal are randomly distributed among the atoms of another. Such alloys are typically formed by atoms with metallic radii that are within approximately 15 percent of each other.

- Due to the similar radii and other characteristics of transition metals, these metals readily form alloys. The resulting alloys are often hard and possess high melting points.
- Notable examples include ferrous alloys, where metals like chromium, vanadium, tungsten, molybdenum, and manganese are utilized for the production of various steels and stainless steel.
- Additionally, alloys formed by combining transition metals with non-transition metals, such as brass (copper-zinc) and bronze (copper-tin), hold considerable industrial importance
Practice Questions
Q.1 Give the general electronic configuration valence shell of 'd' block elements
Ans: The generalised electronic configuration of these elements is (n-1) d1–10 ns1–2.
- Transition elements are those elements whose two outermost shells are incomplete.
- These elements have partially filled d-subshell in the ground state or any of their common oxidation state and are commonly referred to as d-block transition elements.
- The d-block elements are categorised as 1st series transition elements, 2nd series transition elements, 3rd series transition elements and 4th series transition elements.
The examples are:- Cu, Zn, Ag, Cd, Au, Hg, etc.
Q.2 Which of the following is not the characteristic of a transition element?
- Are hard and have high densities.
- Form coloured ions and compounds.
- Show fixed oxidation state.
- More than one of the above.
Ans: The correct answer is option 3, i.e. Show fixed oxidation state.
- Transition elements are those elements whose two outermost shells are incomplete.
- These elements have partially filled d-subshell in the ground state or any of their common oxidation state and are commonly referred to as d-block transition elements.
- The d-block elements are categorized as 1st series transition elements, 2nd series transition elements, 3rd series transition elements, and 4th series transition elements.
- Examples are:- Cu, Zn, Ag, Cd, Au, Hg, etc.
- The f-block elements are termed inner transition elements.
- Some characteristics of Transition elements are;
They are hard and have high densities.
They always form colored ions and compounds.
They have high melting and boiling points.
They have more than one oxidation state.
|
135 videos|348 docs|182 tests
|
FAQs on Configuration & Properties of d-Block Elements - Chemistry Class 12 - NEET
| 1. What are the d-block elements and where are they located in the periodic table? |  |
| 2. What is the general electronic configuration of d-block elements? |  |
| 3. What are the physical properties of transition elements? |  |
| 4. How do the atomic and ionic radii vary among d-block elements? |  |
| 5. What trends are observed in the standard electrode potentials of M2+/M and M3+/M2+ for d-block elements? |  |

|
Explore Courses for NEET exam
|

|

 Position on the periodic table: Generally, transition elements are found in the d-block of the modern periodic table, specifically in groups 3 to 12. These groups include elements like titanium, iron, and copper.
Position on the periodic table: Generally, transition elements are found in the d-block of the modern periodic table, specifically in groups 3 to 12. These groups include elements like titanium, iron, and copper. 
























