Gibbs Energy and Redox Reactions | Chemistry Optional Notes for UPSC PDF Download
Introduction
- Changes in reaction conditions can have a tremendous effect on the course of a redox reaction. For example, under standard conditions, the reaction of Co(s) with Ni2+(aq) to form Ni(s) and Co2+(aq) occurs spontaneously, but if we reduce the concentration of Ni2+ by a factor of 100, so that [Ni2+] is 0.01 M, then the reverse reaction occurs spontaneously instead.
- The relationship between voltage and concentration is one of the factors that must be understood to predict whether a reaction will be spontaneous.
The Relationship between Cell Potential & Gibbs Energy
- Electrochemical cells convert chemical energy to electrical energy and vice versa. The total amount of energy produced by an electrochemical cell, and thus the amount of energy available to do electrical work, depends on both the cell potential and the total number of electrons that are transferred from the reductant to the oxidant during the course of a reaction. The resulting electric current is measured in coulombs (C), an SI unit that measures the number of electrons passing a given point in 1 s. A coulomb relates energy (in joules) to electrical potential (in volts). Electric current is measured in amperes (A); 1 A is defined as the flow of 1 C/s past a given point (1 C = 1 A·s):
1 J/1 V = 1 C = A.s (20.5.1) - In chemical reactions, however, we need to relate the coulomb to the charge on a mole of electrons. Multiplying the charge on the electron by Avogadro’s number gives us the charge on 1 mol of electrons, which is called the faraday (F), named after the English physicist and chemist Michael Faraday (1791–1867):
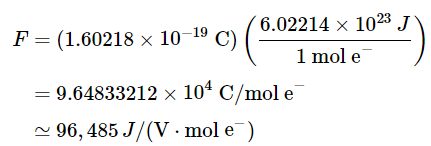 (20.5.2), (20.5.3), (20.5.4)
(20.5.2), (20.5.3), (20.5.4) - The total charge transferred from the reductant to the oxidant is therefore nF, where n is the number of moles of electrons.
Michael Faraday (1791–1867)
Faraday was a British physicist and chemist who was arguably one of the greatest experimental scientists in history. The son of a blacksmith, Faraday was self-educated and became an apprentice bookbinder at age 14 before turning to science. His experiments in electricity and magnetism made electricity a routine tool in science and led to both the electric motor and the electric generator. He discovered the phenomenon of electrolysis and laid the foundations of electrochemistry. Most of the specialized terms introduced in this chapter (electrode, anode, cathode, and so forth) are due to Faraday. In addition, he discovered benzene and invented the system of oxidation state numbers that we use today. Faraday is probably best known for “The Chemical History of a Candle,” a series of public lectures on the chemistry and physics of flames.
- The maximum amount of work that can be produced by an electrochemical cell (wmax) is equal to the product of the cell potential (E∘cell) and the total charge transferred during the reaction (nF):
wmax = nF Ecell (20.5.5) - Work is expressed as a negative number because work is being done by a system (an electrochemical cell with a positive potential) on its surroundings.
- The change in free energy (ΔG) is also a measure of the maximum amount of work that can be performed during a chemical process (ΔG = wmax). Consequently, there must be a relationship between the potential of an electrochemical cell and ΔG; this relationship is as follows:
ΔG = −nFEcell - A spontaneous redox reaction is therefore characterized by a negative value of ΔG and a positive value of E∘cell, consistent with our earlier discussions. When both reactants and products are in their standard states, the relationship between ΔG° and E∘cell is as follows:
ΔG° = −nF E∘cell (20.5.7) - A spontaneous redox reaction is characterized by a negative value of ΔG°, which corresponds to a positive value of E°cell.
Solved Example
Example 1: Suppose you want to prepare elemental bromine from bromide using the dichromate ion as an oxidant. Using the data in Table P2, calculate the free-energy change (ΔG°) for this redox reaction under standard conditions. Is the reaction spontaneous?
Given: redox reaction
Asked for: ΔGo for the reaction and spontaneity
Strategy: (a) From the relevant half-reactions and the corresponding values of Eo, write the overall reaction and calculate E∘cell.
(b) Determine the number of electrons transferred in the overall reaction. Then use Equation 20.5.7 to calculate ΔGo. If ΔGo is negative, then the reaction is spontaneous.
Ans: (a) As always, the first step is to write the relevant half-reactions and use them to obtain the overall reaction and the magnitude of Eo. From Table P2, we can find the reduction and oxidation half-reactions and corresponding Eo values: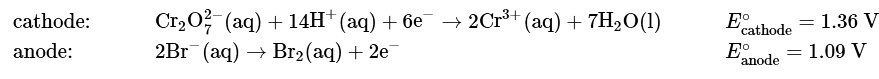
To obtain the overall balanced chemical equation, we must multiply both sides of the oxidation half-reaction by 3 to obtain the same number of electrons as in the reduction half-reaction, remembering that the magnitude of Eo is not affected: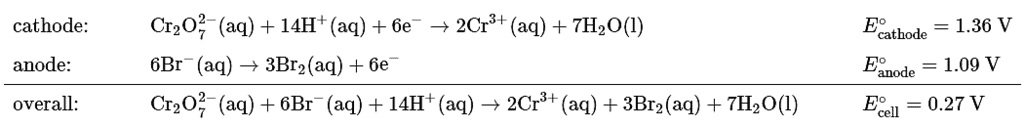
(b) We can now calculate ΔG° using Equation 20.5.7. Because six electrons are transferred in the overall reaction, the value of n is 6: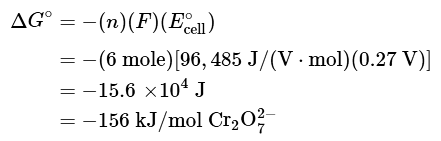
Thus ΔGo is −168 kJ/mol for the reaction as written, and the reaction is spontaneous.
Potentials for the Sums of Half-Reactions
- Although Table P2 list several half-reactions, many more are known. When the standard potential for a half-reaction is not available, we can use relationships between standard potentials and free energy to obtain the potential of any other half-reaction that can be written as the sum of two or more half-reactions whose standard potentials are available. For example, the potential for the reduction of Fe3+(aq) to Fe(s) is not listed in the table, but two related reductions are given:
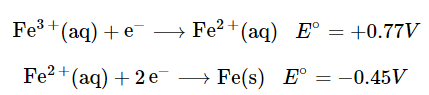 (20.5.8), (20.5.9)
(20.5.8), (20.5.9) - Although the sum of these two half-reactions gives the desired half-reaction, we cannot simply add the potentials of two reductive half-reactions to obtain the potential of a third reductive half-reaction because Eo is not a state function. However, because ΔGo is a state function, the sum of the ΔGo values for the individual reactions gives us ΔGo for the overall reaction, which is proportional to both the potential and the number of electrons (n) transferred. To obtain the value of Eo for the overall half-reaction, we first must add the values of ΔGo(=−nFEo) for each individual half-reaction to obtain ΔGo for the overall half-reaction:
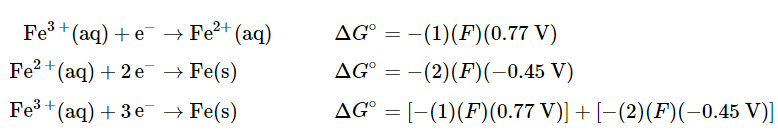
- Solving the last expression for ΔG° for the overall half-reaction,
ΔG∘ = F[(−0.77V) + (−2)(−0.45V)] = F(0.13V) (20.5.10), (20.5.10)
Three electrons (n=3) are transferred in the overall reaction, so substituting into Equation 20.5.7 and solving for Eo gives the following: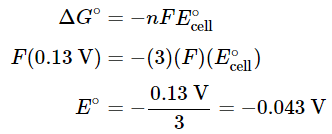
- This value of Eo is very different from the value that is obtained by simply adding the potentials for the two half-reactions (0.32 V) and even has the opposite sign. Values of Eo for half-reactions cannot be added to give Eo for the sum of the half-reactions; only values of ΔGo = −nFE∘cell for half-reactions can be added.
The Relationship between Cell Potential & the Equilibrium Constant
We can use the relationship between ΔG∘ and the equilibrium constant K, to obtain a relationship between E∘cell and K. Recall that for a general reaction of the type αA + bB → cC + dD, the standard free-energy change and the equilibrium constant are related by the following equation:
ΔG° = −RT ln K (20.5.11)
Given the relationship between the standard free-energy change and the standard cell potential (Equation 20.5.7), we can write
−nF E∘cell = −RT ln K (20.5.12)
Rearranging this equation,
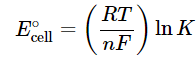 (20.5.13)
(20.5.13)
For T = 298K, Equation 20.5.13 can be simplified as follows:
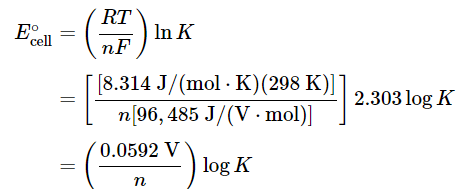 (20.5.14), (20.5.15), (20.5.16)
(20.5.14), (20.5.15), (20.5.16)
Thus E∘cell is directly proportional to the logarithm of the equilibrium constant. This means that large equilibrium constants correspond to large positive values of E∘cell and vice versa.
Solved Example
Example: Use the data in Table P2 to calculate the equilibrium constant for the reaction of metallic lead with PbO2 in the presence of sulfate ions to give PbSO4 under standard conditions. (This reaction occurs when a car battery is discharged.) Report your answer to two significant figures.
Given: redox reaction
Asked for: K
Strategy: (a) Write the relevant half-reactions and potentials. From these, obtain the overall reaction and Eocell.
(b) Determine the number of electrons transferred in the overall reaction. Use Equation 20.5.16 to solve for logK and then K.
Ans: (a) The relevant half-reactions and potentials from Table P2 are as follows:
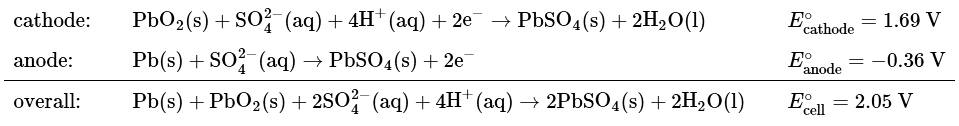
(b) Two electrons are transferred in the overall reaction, so n = 2. Solving Equation 20.5.16 for log K and inserting the values of n and Eo,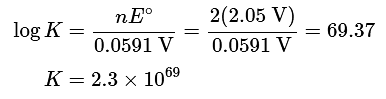
Thus the equilibrium lies far to the right, favoring a discharged battery (as anyone who has ever tried unsuccessfully to start a car after letting it sit for a long time will know).
Figure 20.5.1 summarizes the relationships that we have developed based on properties of the system—that is, based on the equilibrium constant, standard free-energy change, and standard cell potential—and the criteria for spontaneity (ΔG° < 0). Unfortunately, these criteria apply only to systems in which all reactants and products are present in their standard states, a situation that is seldom encountered in the real world. A more generally useful relationship between cell potential and reactant and product concentrations, as we are about to see, uses the relationship between ΔG and the reaction quotient Q.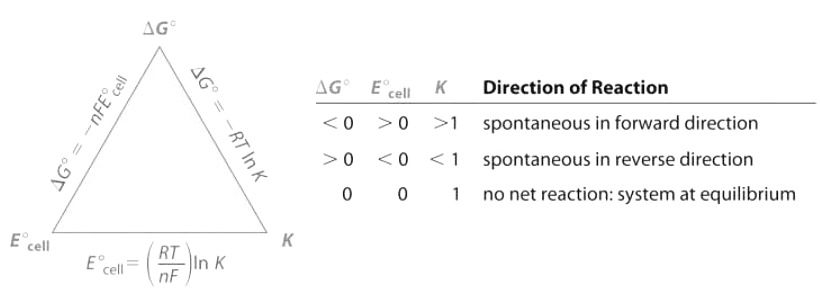
Figure 20.5.1: The Relationships among Criteria for Thermodynamic Spontaneity. The three properties of a system that can be used to predict the spontaneity of a redox reaction under standard conditions are K, ΔG°, and E°cell. If we know the value of one of these quantities, then these relationships enable us to calculate the value of the other two. The signs of ΔG° and E°cell and the magnitude of K determine the direction of spontaneous reaction under standard conditions.
- If delta G is less than zero, E is greater than zero and K is greater than 1 then the direction of the reaction is spontaneous in forward direction. If delta G is greater than zero, E is less than zero and K is less than one then the direction of reaction is spontaneous in reverse direction. If delta G is zero, E is zero and k is one that there is no net reaction and the system is at equilibrium .
Summary
A coulomb (C) relates electrical potential, expressed in volts, and energy, expressed in joules. The current generated from a redox reaction is measured in amperes (A), where 1 A is defined as the flow of 1 C/s past a given point. The faraday (F) is Avogadro’s number multiplied by the charge on an electron and corresponds to the charge on 1 mol of electrons. The product of the cell potential and the total charge is the maximum amount of energy available to do work, which is related to the change in free energy that occurs during the chemical process. Adding together the ΔG values for the half-reactions gives ΔG for the overall reaction, which is proportional to both the potential and the number of electrons (n) transferred. Spontaneous redox reactions have a negative ΔG and therefore a positive Ecell. Because the equilibrium constant K is related to ΔG, E°cell and K are also related. Large equilibrium constants correspond to large positive values of E°.
FAQs on Gibbs Energy and Redox Reactions - Chemistry Optional Notes for UPSC
| 1. What is the relationship between cell potential and Gibbs energy? |  |
| 2. How can cell potential be used to determine the equilibrium constant? |  |
| 3. What are the potentials for the sums of half-reactions? |  |
| 4. How does Gibbs energy relate to redox reactions? |  |
| 5. How do cell potential and the equilibrium constant relate to each other? |  |















