Group 15 Elements: Nitrogen Family | Chemistry Class 11 - NEET PDF Download
What are Group 15 Elements?
Group 15 elements are also called the Nitrogen family includes nitrogen phosphorus, arsenic, antimony and bismuth elements. The p-block elements are also known as the Representative Elements which is placed on the right side of the main periodic table. The 15 group of the Periodic Table consists of nitrogen, phosphorus, arsenic, antimony and bismuth. These elements are known as pnicogens and their compounds as pniconides.

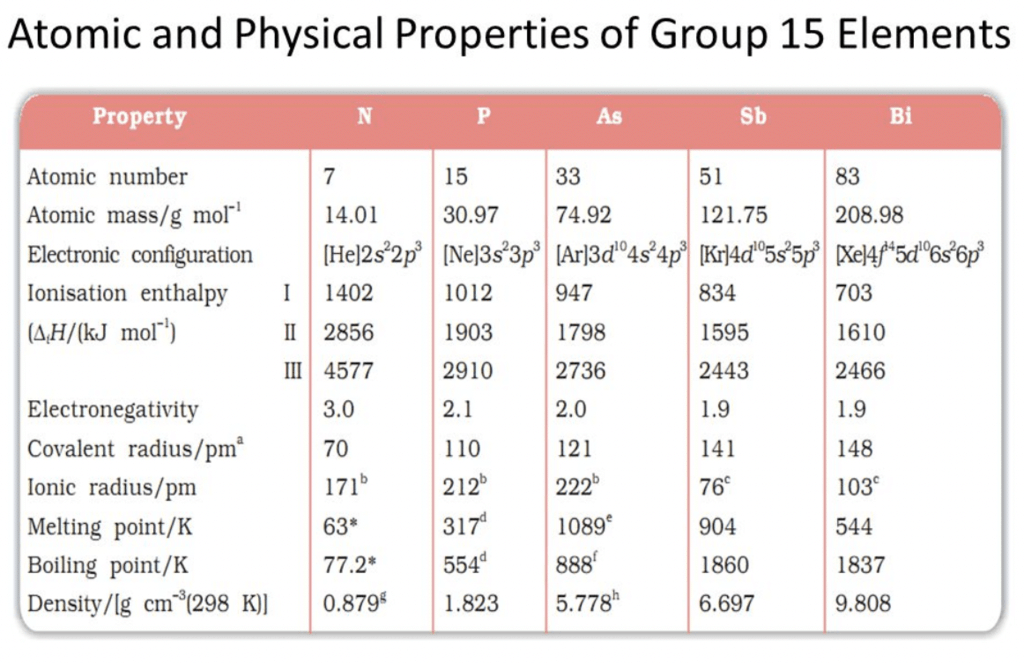
Physical Properties of Group 15 Elements
Electronic configuration:
Their valence shell electronic configuration is ns2 np3
Metallic character:
N and P are non-metals, As and Sb are metalloids and Bi is metal.Physical state:
Nitrogen is the first element after hydrogen which is diatomic gas in native form. All other elements in the group are solids.Atomicity:
N2 is diatomic while others are triatomic.Melting and boiling points:
The melting point increases from nitrogen to arsenic. The boiling points increase regularly on moving down the group.Density:
It increases down the group.Atomic radii:
It increases with an increase in atomic number as we go down the group.Allotropy:
All the elements (except Bi) exhibit allotropy. Nitrogens – α nitrogen, β – nitrogen.
Phosphorus – White, red, black
Arsenic – Grey, yellow, black
Antimony – Metallic yellow (explosive)Oxidation state:
 Nitrogen has a wide range of oxidation states. The stability of the +3 oxidation state increases and the stability of the +5 oxidation state decreases on moving down the group due to the inert pair effect.Question for Group 15 Elements: Nitrogen FamilyTry yourself:What catalyst is used for oxidation of ammonia to produce nitric acid?View Solution
Nitrogen has a wide range of oxidation states. The stability of the +3 oxidation state increases and the stability of the +5 oxidation state decreases on moving down the group due to the inert pair effect.Question for Group 15 Elements: Nitrogen FamilyTry yourself:What catalyst is used for oxidation of ammonia to produce nitric acid?View SolutionThe ionization enthalpy:
The Ionisation energy of nitrogen is very high due to its small size and half-filled highly stable configuration. The ionization energy decreases down the group.Electronegativity:
It decreases from nitrogen to bismuth.Catenation:
‘They exhibit the property of catenation but to a lesser extent due to weak E – E bond than 14 group elements.Reactivity:
Elemental nitrogen is highly unreactive because of its strong triple bond. (almost as inert as noble gases). White phosphorus is extremely reactive and is kept in water. It is inflammable and can be ignited at 45°C.
Chemical Properties of Group 15 Elements
Hydrides:
All the elements of this group form hydrides of the type EH3, which are covalent and pyramidal in shape. Some properties follow the order as mentioned: These properties are:
These properties are:
(i) Thermal stability,
(ii) Basic strength,
(iii) Solubility in water,
(iv) Bond angle NH3 (107.4°); PH3 (92°),AsH3 (91° ), SbH3(90° ),
(v) Strength of M – H bond
Some properties follow the order: NH3 < PH3 < AsH3 < SbH3 < BiH3
These properties are :
(i) Reducing character
(ii) Covalent character
(iii) Rate of combustionHalides:
All the elements of this group form trihalides, MX3 and except nitrogen all form pentahalides, MX5, e.g., NCi3, NI3, PCI3, BiCI3, AsCI3, PCl5 etc. Trihalides (except of N) behaves as Lewis acid and the order of their strength is PCl3 > AsCl3 > SbCl3. Trihalides of N behave as Lewis base and has the following order of strength-NF3 < NCl3 < NBr3 < NI3.
NCl3 is an explosive compound.Oxides:
All the elements of this group form oxides of the type M2O3 and M2O5. N2O5 and N2O4 are strongly acidic, whereas, NO and N2O are neutral.
N2O5 and N2O4 are strongly acidic, whereas, NO and N2O are neutral.
P4O10 is also strongly acidic.
As4O6 is called white arsenic and is a poison.
The acidic strength of pentoxides and trioxides decrease on moving down the group, i.e., N2O5 > P2O5 > As2O5 > Sb2O5.
BiOCl is called pearl white.
Compounds of Nitrogen
Dinitrogen (N2)
Preparation: Properties:
Properties:
(i) Nitrogen does not react with alkali metals except Li but reacts with alkaline earth metals to give metal nitride. (ii) Reaction with oxygen
(ii) Reaction with oxygen (iii) Reaction with non-metals
(iii) Reaction with non-metals (iv) Reaction with CaC2
(iv) Reaction with CaC2 Uses:
Uses:
Liquid N2 is used as a refrigerant. N2 is used in the manufacture of HNO2, NH2, CaCN2(calcium cyanamide) and other nitrogenous compounds. It is used for filling electric bulbs.Question for Group 15 Elements: Nitrogen FamilyTry yourself:Why does nitrogen show anomalous properties with respect to other elements in group 15?View SolutionAmmonia (NH3)
Preparation:
(i) Lab method: 2NH4Cl + Ca(OH)2 → CaCI2 + 2NH3 + 2H2O
(ii) Haber’s process
Properties:
(i). It is a colourless gas with a characteristic pungent odour. It is extremely soluble in water due to H – bonding.
(ii). It is a strong Lewis base and used in the metal ion detection as- 3. Reaction with chlorine:
3. Reaction with chlorine:
When NH3 is in excess, N2 is the main product.
8NH3 + 3Cl2 → 6NH4Cl + N3
When Cl2 is in excess, NCl3 is the main product.
NH3 + 3Cl2 → NCl3 + 3HCl
4. Reaction with Nessler’s reagent:

Uses:
It is used as a refrigerant and to produce various nitrogenous fertilizers.Question for Group 15 Elements: Nitrogen FamilyTry yourself:How many unshared pair of electrons does an ammonia molecule have?View SolutionOxides of Nitrogen
 NO2 contains an odd number of valence electrons. On dimerisation, it is converted to a stable N2O4 molecule with an even number of electrons.Question for Group 15 Elements: Nitrogen FamilyTry yourself:Which of the following is not an alternative name of dinitrogen trioxide?View Solution
NO2 contains an odd number of valence electrons. On dimerisation, it is converted to a stable N2O4 molecule with an even number of electrons.Question for Group 15 Elements: Nitrogen FamilyTry yourself:Which of the following is not an alternative name of dinitrogen trioxide?View SolutionNitric acid (HNO3):
It is a stronger acid than H3PO4.
Preparation:
(i) Lab method: NaNO3 + H2SO4 (conc.) → NaHSO4 + HNO3
(ii) Ostwald’s process
Physical properties: It is a syrupy, colourless, pungent liquid usually available as 68 % and 15.7 M aqueous solution is often yellow due to small concentrations of NO2.Question for Group 15 Elements: Nitrogen FamilyTry yourself:What is the catalyst used in the industrial manufacture of nitric acid?View SolutionChemical reactions:
(i). Action of nitric acid on zinc under different conditions: (ii). Action of nitric acid on copper under different conditions:
(ii). Action of nitric acid on copper under different conditions: (iii). Reaction with non-metals:
(iii). Reaction with non-metals: (iv). Brown ring test of nitrate:
(iv). Brown ring test of nitrate: (v). Metals like Fe. Cr. Ni, AI or Co becomes inactive or passive due to stable oxide layers.
(v). Metals like Fe. Cr. Ni, AI or Co becomes inactive or passive due to stable oxide layers.
Structure of nitric acid:
Uses: It is used
1.In the manufacturing of fertilizers.
2. For purification of silver and gold.
3. In the manufacturing of explosives and as oxidising agent.
4. As nitrating reagent.Question for Group 15 Elements: Nitrogen FamilyTry yourself:How many allotropes does nitrogen have?View Solution
Phosphorus & its Allotropic Forms
Phosphorus is found in many allotropic forms, the important ones being white, red and black.
Some Points of Distinction Between White and Red Phosphorus

 Black phosphorus is formed when red phosphorus is heated in a sealed tube at 803 K. It does not oxidise in air. The black phosphorous is also of further 2 types: α and β.
Black phosphorus is formed when red phosphorus is heated in a sealed tube at 803 K. It does not oxidise in air. The black phosphorous is also of further 2 types: α and β.
Matchbox side contains red P or P2S3+ glue and on tip of a match stick. red P, KClO3 chalk and glue is deposited.
 Types of Black Phosphorous
Types of Black Phosphorous
Chemical properties:
- With non-metals:

- With compounds:

Uses
It is used in matchboxes, explosives, as rat poison, in fertilizers and alloys
Compounds of Phosphorous
Phosphine (PH3)
Preparation It is prepared by following methods: Properties
Properties
(i). It is a colourless gas with a rotten fish-like smell and is highly poisonous. It explodes in contact with traces of oxidising agents like HNO3, Cl2 and Br2 vapours.
3CuSO4 + 2PH3 → CU3P2 + 3H2SO4
3HgCl2 + 2PH3 → Hg3P2 + 6HCl
(ii). Phosphine is weakly basic.
PH3 + HBr → PH+4+ Br–
Uses: It is used to prepare smoke screens in warfare. A mixture of CaC2 and Ca3P2 is used in Holme’s signals.Question for Group 15 Elements: Nitrogen FamilyTry yourself:What is the hybridization of phosphine?View SolutionPhosphorus Trichloride (PCl3)
Preparation:
Properties: It is a colourless oily liquid having a pyramidal shape [sp3 – hybridised].

Phosphorus Pentachloride (PCl5)
Preparation:
P4 + 10 Cl2 → 4 PCl5
P4 + 10 SO2CI2 → 4PCl5 + 10 SO2
Structure: PCl5 in gaseous and liquid phases has sp3d – hybridization and its shape is trigonal-bipyramidal. The three equatorial P – Cl bonds are equivalent while the two axial bonds are longer equatorial bonds.
Properties: In solid-state, PCl5 exists as an ionic solid, [PCI4]+ [PCl6]– in which, the cation, [PCI4]+ is tetrahedral and the anion [PCl6]– is octahedral.
Oxoacids of Phosphorus




In toothpaste, CaHPO4.2H2O is added as a mild abrasive and polish agent.
|
127 videos|244 docs|87 tests
|
FAQs on Group 15 Elements: Nitrogen Family - Chemistry Class 11 - NEET
| 1. What are the physical properties of Group 15 elements? |  |
| 2. What are the chemical properties of Group 15 elements? |  |
| 3. What are some common compounds of Nitrogen? |  |
| 4. What are the different allotropic forms of phosphorus? |  |
| 5. What are some common compounds of Phosphorus? |  |

|
Explore Courses for NEET exam
|

|

 Nitrogen has a wide range of oxidation states. The stability of the +3 oxidation state increases and the stability of the +5 oxidation state decreases on moving down the group due to the inert pair effect.
Nitrogen has a wide range of oxidation states. The stability of the +3 oxidation state increases and the stability of the +5 oxidation state decreases on moving down the group due to the inert pair effect. These properties are:
These properties are: N2O5 and N2O4 are strongly acidic, whereas, NO and N2O are neutral.
N2O5 and N2O4 are strongly acidic, whereas, NO and N2O are neutral. Properties:
Properties: (ii) Reaction with oxygen
(ii) Reaction with oxygen (iii) Reaction with non-metals
(iii) Reaction with non-metals (iv) Reaction with CaC2
(iv) Reaction with CaC2 Uses:
Uses:
 3. Reaction with chlorine:
3. Reaction with chlorine: NO2 contains an odd number of valence electrons. On dimerisation, it is converted to a stable N2O4 molecule with an even number of electrons.
NO2 contains an odd number of valence electrons. On dimerisation, it is converted to a stable N2O4 molecule with an even number of electrons.
 (ii). Action of nitric acid on copper under different conditions:
(ii). Action of nitric acid on copper under different conditions: (iii). Reaction with non-metals:
(iii). Reaction with non-metals: (iv). Brown ring test of nitrate:
(iv). Brown ring test of nitrate: (v). Metals like Fe. Cr. Ni, AI or Co becomes inactive or passive due to stable oxide layers.
(v). Metals like Fe. Cr. Ni, AI or Co becomes inactive or passive due to stable oxide layers.


 Properties
Properties




















