Huckel's Rule: What Does 4n+2 Mean? | Chemistry Optional Notes for UPSC PDF Download
Stop Looking For “n” In A Molecule, Because You’re Looking For The Wrong Thing
The other night a student came to me with a question about aromaticity.
“There’s one thing I don’t get”, she said. “They say a molecule has to have 4n+2 electrons to be aromatic. How do you find ‘n’ ?”
Teachable moment!
These are aromatic molecules containing (4n+2) pi electrons, where “n” is a natural number
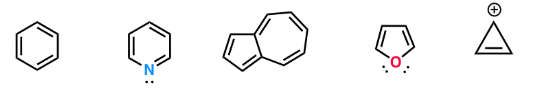 Count the pi-electrons - don’t look for “n” !
Count the pi-electrons - don’t look for “n” !
In the [4n+2] Rule (“Huckel’s Rule”), “n” Is Not A Characteristic of The Molecule!
“n” is not a characteristic of the molecule! Let me explain.
In order for a molecule to be aromatic, it has to have the following characteristics:
- It must be cyclic
- It must be conjugated (i.e. all atoms around the ring must be able to participate in π-bonding through resonance)
- It must be flat
In Huckel’s Rule, The Formula (4n+2) Is An Algebraic Expression Of The Series 2, 6, 10, 14… Where ‘n’ Is A Natural Number
- For n = 0, we get (4 × 0 + 2) = 2
- For n = 1, we get (4 × 1 + 2) = 6
- For n = 2, we get (4 × 2 + 2) = 10
- For n = 3, we get (4 × 3 + 2) = 14
- For n = 4, we get (4 × 4 + 2) = 18
Summary: “n” Comes From Algebra, NOT From Chemistry
FAQs on Huckel's Rule: What Does 4n+2 Mean? - Chemistry Optional Notes for UPSC
| 1. What is the [4n 2] rule in Huckel's rule? |  |
| 2. What does 'n' represent in the [4n 2] rule? |  |
| 3. Is 'n' a characteristic of the molecule in Huckel's rule? |  |
| 4. How can the [4n 2] rule be applied to determine aromaticity? |  |
| 5. What is the significance of the [4n 2] rule in organic chemistry? |  |

|
Explore Courses for UPSC exam
|

|
















