Hydrocarbons: Classification, Properties, Preparation & Uses | Chemistry Class 11 - NEET PDF Download
What are Hydrocarbons?
Hydrocarbons are organic compounds that are entirely made up of only two kinds of atoms – carbon and hydrogen.
- Typically, hydrocarbons are colourless gases that have very weak odours. Hydrocarbons can feature simple or relatively complex structures and can be generally classified into four subcategories, namely alkanes, alkenes, alkynes, and aromatic.
- The study of hydrocarbons can provide insight into the chemical properties of other functional groups and their preparation.
Classification of Hydrocarbons
Hydrocarbons are organic compounds composed of carbon and hydrogen atoms. They can be classified into several groups based on their structure and properties.
The main classifications of hydrocarbons are as follows:
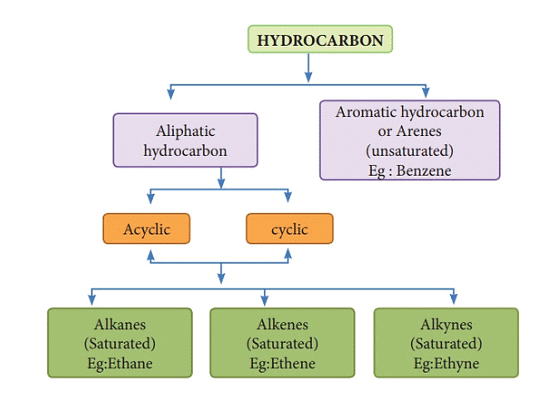 Classification of Hydrocarbons
Classification of Hydrocarbons
1. Acyclic or Open Chain Hydrocarbons
Acyclic hydrocarbons, are hydrocarbons that do not form a cyclic or ring structure. They consist of straight or branched chains of carbon atoms. They can be further classified into two main types: Saturated and Unsaturated.
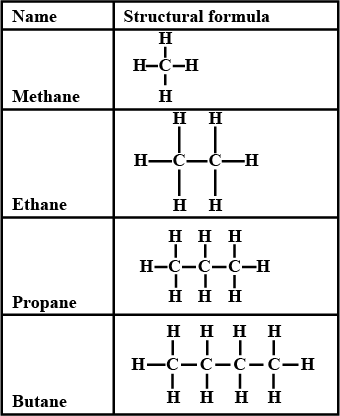
(i) Saturated Hydrocarbons:
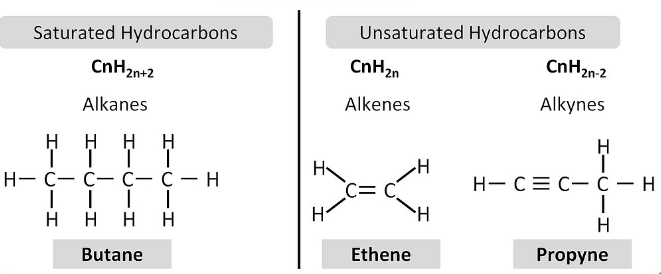
In these compounds, carbon-carbon atoms and carbon-hydrogen atoms are held together by single bonds. These single-bonded compounds are the simplest hydrocarbons. These types of hydrocarbons don’t have double or triple bonds. In terms of hybridization, they have sp3 hybridised carbon atom with no sp2 or sp hybridised carbon atoms. They are together called alkanes which have a general formula CnH2n+2
 Saturated Hydrocarbons
Saturated Hydrocarbons
(ii) Unsaturated Hydrocarbons:
These compounds consist of a single, double or triple bond between carbon-carbon atoms. The double-bonded compounds are called alkenes and the triple bonded compounds are called alkynes. The general formula for alkenes is CnH2n and for alkynes the general formula is CnH2n-2.
 Unsaturated Hydrocarbons
Unsaturated Hydrocarbons
Q. What is the Correct Definition of Aliphatic Organic Compounds?
Students may occasionally find the definition of aliphatic organic compounds confusing. To clarify, aliphatic organic compounds refer to a group of organic compounds composed of carbon (C) and hydrogen (H) atoms arranged in open chains, branched chains, or non-aromatic rings. Importantly, these compounds do not contain any aromatic rings (specifically, benzene rings). Therefore, Aliphatic compounds encompass both open chain structures and cyclic structures that lack aromatic characteristics.
2. Cyclic or Closed Chain Hydrocarbons
Cyclic or closed-chain hydrocarbons can be further divided into two main categories: alicyclic and aromatic hydrocarbons.
Let's Discuss them individually:
(i) Alicyclic hydrocarbons
Alicyclic compounds are organic compounds that are both aliphatic and cyclic. These are the saturated or unsaturated hydrocarbons containing non-aromatic rings of carbon atoms. Alicyclic compounds may have one or more aliphatic side chains attached. Alicyclic compounds do not have π electron delocalization or aromatic character. Below are some examples of Alicyclic compounds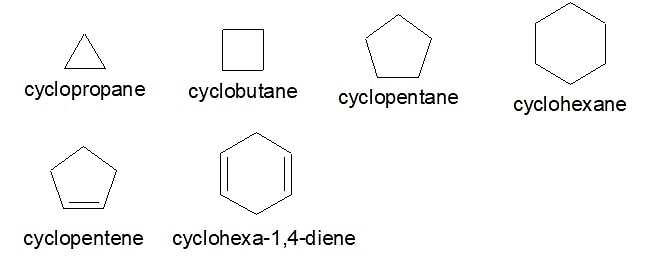 Examples of Alicyclic Compounds
Examples of Alicyclic Compounds
A) Saturated Cyclic Hydrocarbons (Cycloalkanes):
Saturated cyclic hydrocarbons, also known as cycloalkanes, are a specific type of alicyclic hydrocarbons. They are characterized by a closed-ring structure consisting of carbon atoms bonded to each other through single bonds. Cycloalkanes are saturated compounds, meaning that each carbon atom in the ring is bonded to the maximum number of hydrogen atoms.
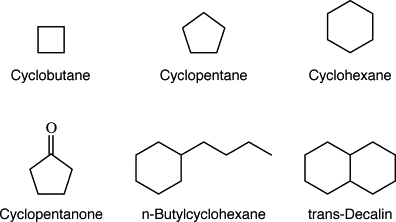 Cycloalkanes
Cycloalkanes
B) Unsaturated Cyclic Hydrocarbons (Cycloalkenes and Cycloalkynes):
Unsaturated cyclic hydrocarbons are cyclic compounds that contain double or triple bonds within the ring structure. They are classified into two main types: cycloalkenes and cycloalkynes.
- Cycloalkenes:
Cycloalkenes are unsaturated cyclic hydrocarbons that contain at least one carbon-carbon double bond within the ring. The general formula for cycloalkenes is CnH2n, where "n" represents the number of carbon atoms in the ring. The presence of the double bond introduces unsaturation and reduces the number of hydrogen atoms compared to the corresponding cycloalkane. Examples of cycloalkenes include cyclopropene (C3H4), cyclobutene (C4H6), cyclopentene (C5H8), and cyclohexene (C6H10).
 Cycloalkenes
Cycloalkenes
- Cycloalkynes:
Cycloalkynes are unsaturated cyclic hydrocarbons that contain at least one carbon-carbon triple bond within the ring. The general formula for cycloalkynes is CnH2n-2, where "n" represents the number of carbon atoms in the ring. The presence of the triple bond reduces the number of hydrogen atoms even further compared to cycloalkenes. Examples of cycloalkynes include cyclopropyne (C3H2), cyclobutyne (C4H4), cyclopentyne (C5H6), and cyclohexyne (C6H8).
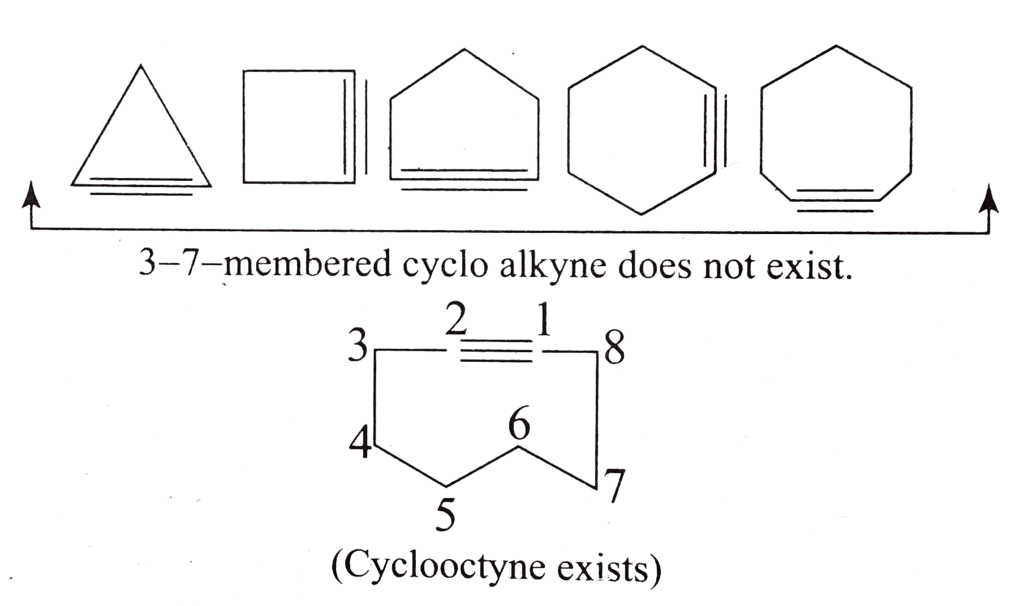 Cycloalkynes
Cycloalkynes
(ii) Aromatic Hydrocarbons: Benzenoid and Non-Benzenoid
Aromatic hydrocarbons are a class of compounds with a specific cyclic structure called an aromatic or benzene ring. They can be classified into benzenoid and non-benzenoid aromatic compounds.
- Benzenoid aromatic hydrocarbons, like benzene, have a hexagonal ring of carbon atoms with alternating double bonds. They are highly stable and undergo electrophilic aromatic substitution reactions. Benzenoid aromatics are used in dyes, pharmaceuticals, and chemicals.
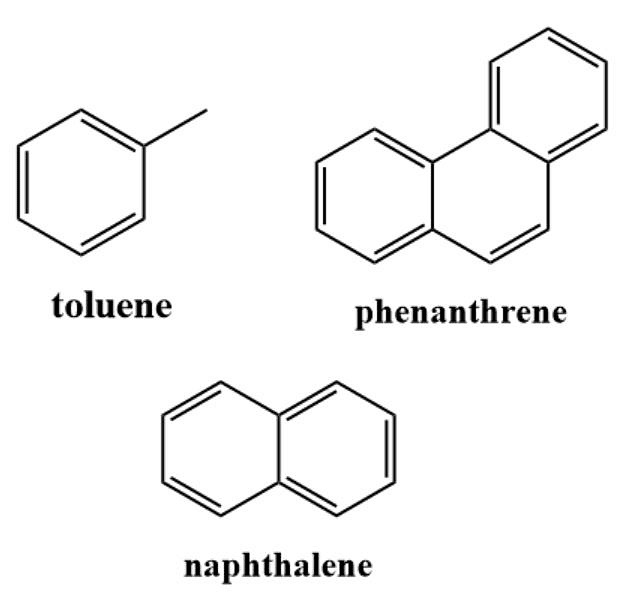 Benzenoid Aromatic Hydrocarbons
Benzenoid Aromatic Hydrocarbons - Non-benzenoid aromatic hydrocarbons have aromatic characteristics but don't have a typical benzene ring. They may have fused rings or incorporate heteroatoms. Examples include naphthalene, pyridine, and furan. Non-benzenoid aromatics are used in dyes, pharmaceuticals, and fragrances.
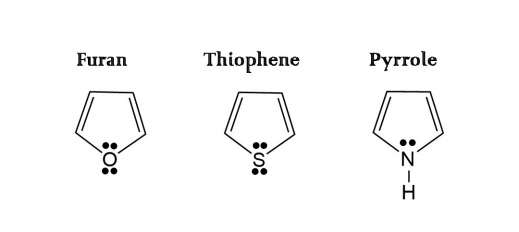 Non-benzenoid aromatic hydrocarbons
Non-benzenoid aromatic hydrocarbons
Both types of aromatic hydrocarbons possess delocalized electrons and exhibit aromatic properties.
Properties of Hydrocarbons
Hydrocarbons, being organic compounds composed solely of carbon and hydrogen atoms, exhibit several key properties. Let's explore these properties in detail:
Physical Properties of Hydrocarbons
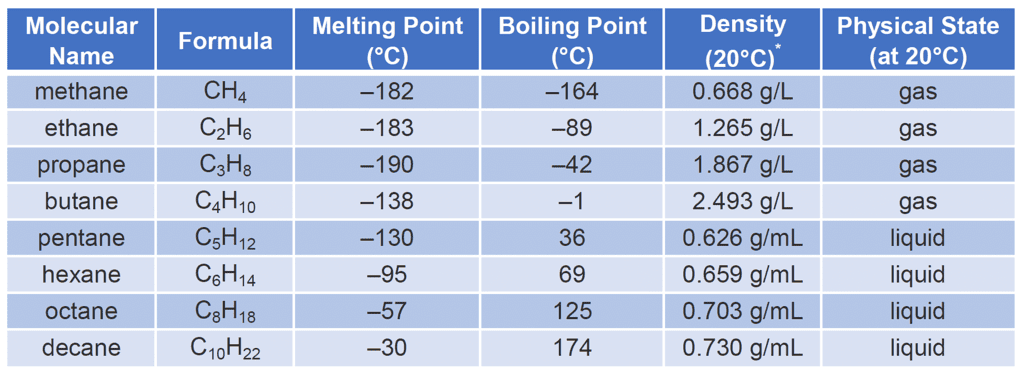
- State of Matter: Most hydrocarbons are typically found in the liquid or gas state at room temperature, depending on their molecular weight. Lighter hydrocarbons with lower molecular weights, such as methane and ethane, are gases, while heavier hydrocarbons, such as hexane and higher, are liquids. However, certain hydrocarbons, like longer-chain alkanes, can be solid at room temperature.
- Melting and Boiling Points: Alkanes generally have low boiling and melting points owing to their weak Vanderwal interaction.
The boiling point depends on the following factors:
(i) Molecular mass
(ii) Branching
Alkanes have high molecular mass and high boiling points. For example, C2H6 has more boiling point than CH4.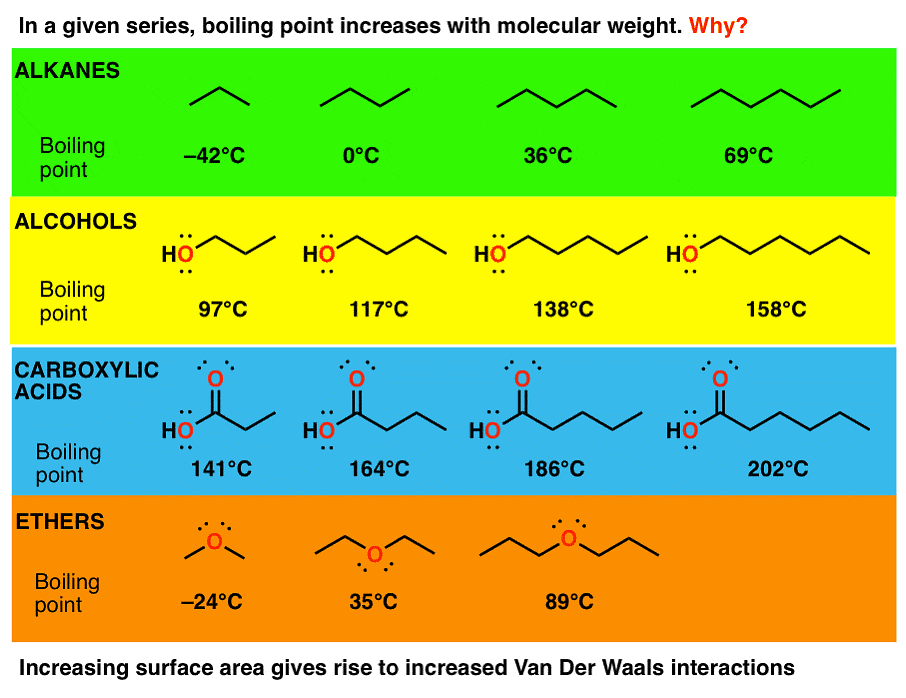 Alkanes that have the same molecular mass but have a different number of branches, the one with less branching has a more boiling point. This is because Vanderwal’s force becomes weak as the area increases.
Alkanes that have the same molecular mass but have a different number of branches, the one with less branching has a more boiling point. This is because Vanderwal’s force becomes weak as the area increases. 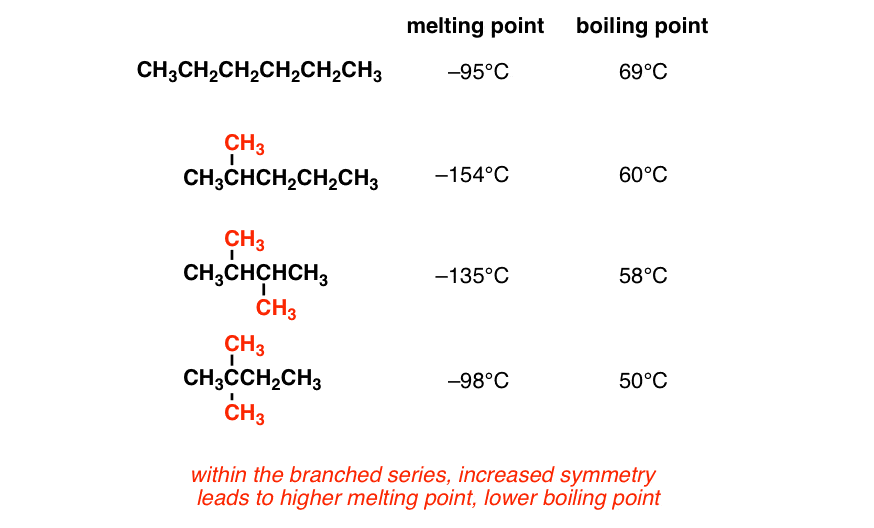 Branching and its affect on Melting and Boiling pointFor example, CH3-CH2-CH2-CH3 has more boiling point. Alkanes are very feebly soluble in water, but they are soluble in non-polar solvents such as Benzene, CCl4, etc.
Branching and its affect on Melting and Boiling pointFor example, CH3-CH2-CH2-CH3 has more boiling point. Alkanes are very feebly soluble in water, but they are soluble in non-polar solvents such as Benzene, CCl4, etc. - Solubility: Hydrocarbons are generally nonpolar molecules and are not soluble in polar solvents such as water. However, they are soluble in nonpolar solvents, such as other hydrocarbons, benzene, or diethyl ether. The solubility of hydrocarbons in water can be enhanced by incorporating functional groups that increase polarity, such as hydroxyl (-OH) or carboxyl (-COOH) groups.
- Density: The density of hydrocarbons varies depending on their molecular weight and structural arrangement. Generally, liquid hydrocarbons are less dense than water, and lighter hydrocarbons (gases) are less dense than air.
Chemical Properties of Hydrocarbons
- Combustibility: Hydrocarbons are highly flammable substances. They readily undergo combustion reactions in the presence of oxygen to produce carbon dioxide (CO2) and water (H2O) as the primary products. The combustion of hydrocarbons releases a significant amount of energy, making them valuable as fuels.
- Reactivity: Hydrocarbons exhibit varying degrees of reactivity depending on their functional groups and unsaturation. Saturated hydrocarbons, such as alkanes, are relatively unreactive under normal conditions. Unsaturated hydrocarbons, such as alkenes and alkynes, are more reactive due to the presence of double or triple bonds, allowing them to undergo addition reactions with electrophiles.
- Halogenation: Hydrocarbons can undergo halogenation reactions in the presence of halogens, such as chlorine (Cl2) or bromine (Br2). This reaction involves the substitution of hydrogen atoms in the hydrocarbon with halogen atoms, resulting in the formation of alkyl halides.
- Oxidation: Depending on their functional groups and degree of saturation, hydrocarbons can undergo oxidation reactions. For example, alkanes are relatively inert to oxidation, while alkenes can be oxidized to form diols or carboxylic acids. Alcohols, which contain hydroxyl (-OH) groups, are prone to oxidation to form aldehydes, ketones, or carboxylic acids.
- Polymerization: Unsaturated hydrocarbons, such as alkenes, can undergo polymerization reactions to form long-chain polymers. This process involves the repeated addition of monomer units to the double bond, resulting in the formation of a polymer with high molecular weight. Examples include the polymerization of ethene to produce polyethylene.Example of Polymerization
- Isomerism: There are two primary types of isomerism, which can be further categorized into different subtypes. These primary types are Structural Isomerism and Stereoisomerism. The classification of different types of isomers is illustrated below.
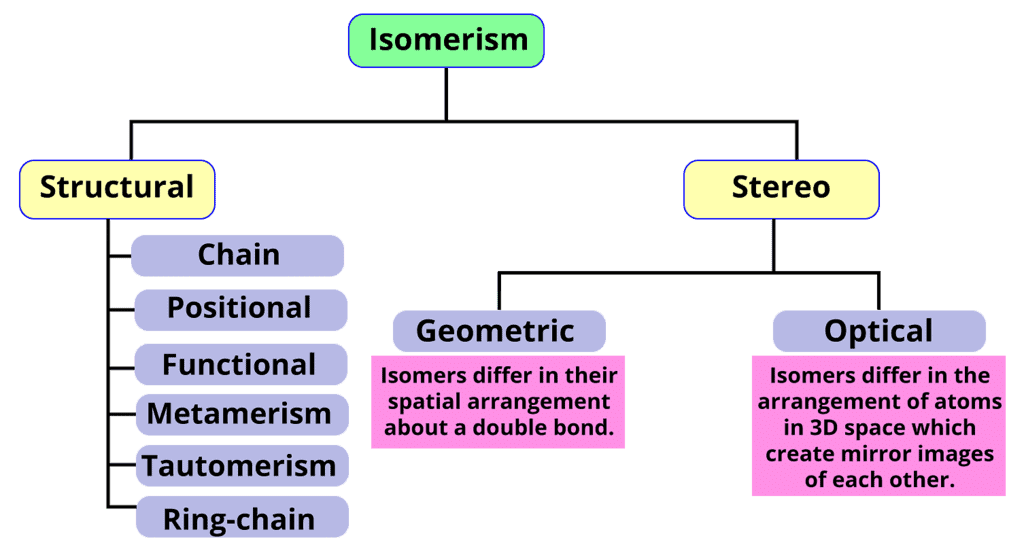 Isomerism in Hydrocarbons
Isomerism in Hydrocarbons
(i) Structural Isomers
Structural isomers have the same formula but different atom arrangements. They exhibit different connectivities based on how their atoms are assembled.- Chain isomerism: It involves linear and branched chains with the same formula. For example, n-butane and isobutane (2-methylpropane) are chain isomers with the formula C4H12.
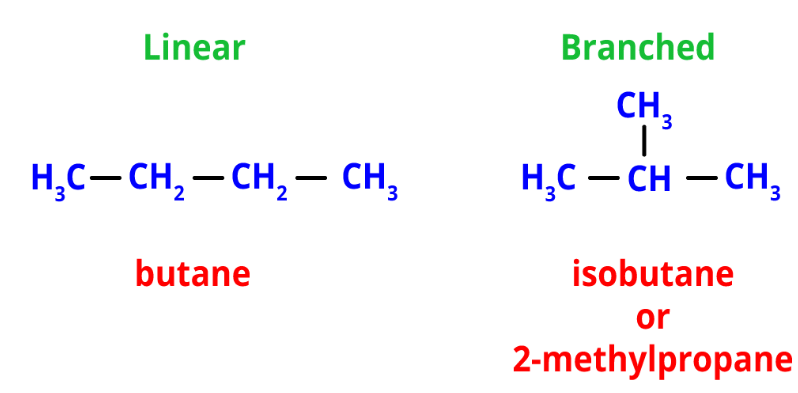 Chain Isomers of Butane
Chain Isomers of Butane - Position isomerism: It arises from different positions of side chains or functional groups on the parent chain. For instance, propyl chloride and isopropyl chloride are position isomers with the formula C3H7Cl.
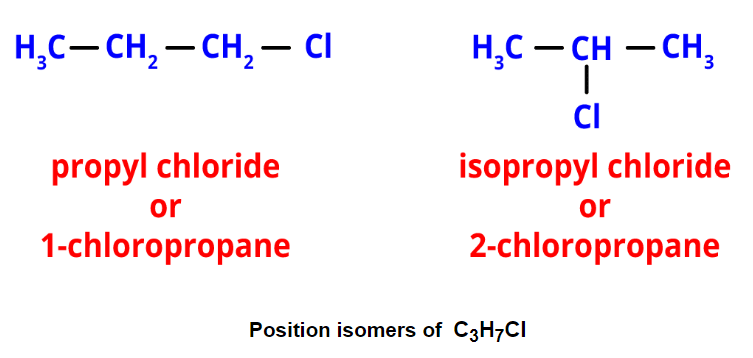
- Functional isomerism: It results from different functional groups while having the same formula. An example is ethyl alcohol and dimethyl ether, both with the formula C2H6O but different functional groups.
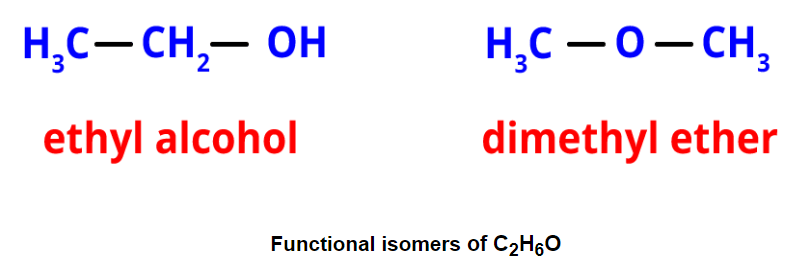
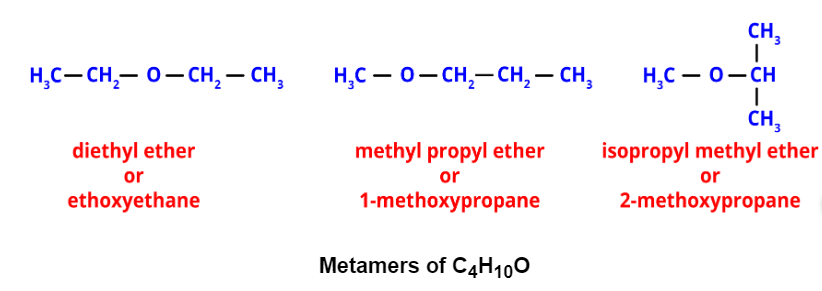
- Metamerism: It occurs when different alkyl groups are attached to the same functional group. Metamers of C4H10O, for example, have different alkyl groups attached to the ether functional group.
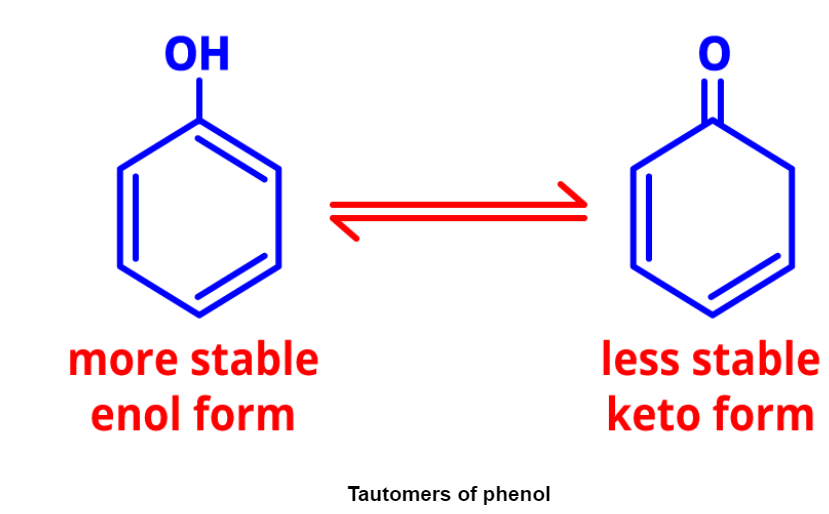
- Tautomerism: It involves dynamic equilibrium between two compounds with the same formula but different functional groups. Tautomers of phenol demonstrate tautomerism.
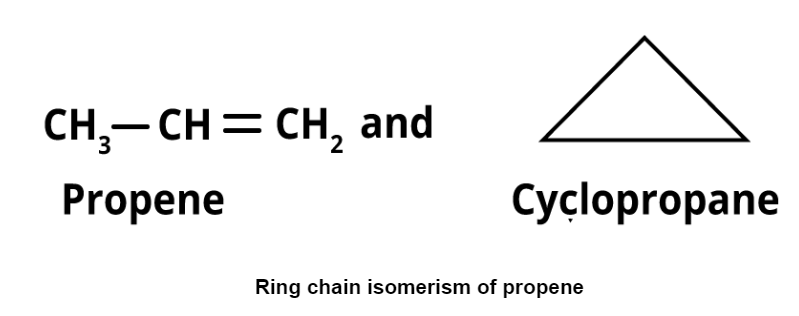
- Ring-chain isomerism: It refers to compounds with the same formula but differing in open chain and cyclic structures. Examples include propene and cyclopropane.
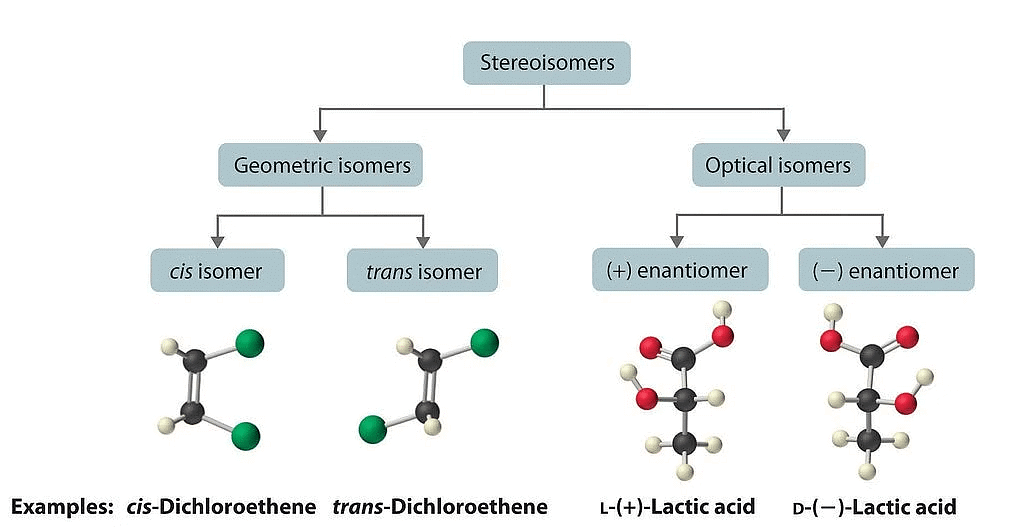
Stereoisomers
Stereoisomerism refers to the phenomenon where compounds have the same molecular formula and connectivity of atoms but differ in their spatial arrangement. There are two main types of stereoisomerism: geometric isomerism and optical isomerism.
 Types of Stereoisomers
Types of Stereoisomers
(i) Geometric Isomerism: Geometric isomerism arises in compounds with restricted rotation around a bond, commonly a carbon-carbon double bond (C=C) or a ring structure. In these cases, the arrangement of substituents around the double bond or within the ring can result in different geometric isomers.
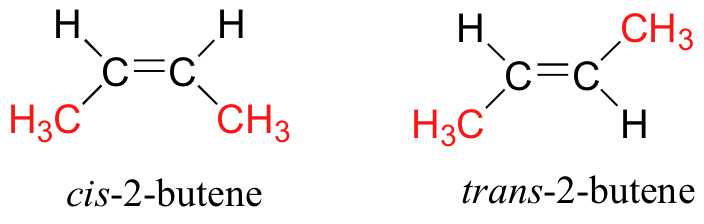
(ii) Optical Isomerism: Optical isomerism occurs when compounds possess chiral centers, which are carbon atoms bonded to four different groups. Chiral molecules cannot be superimposed on their mirror images and exist as pairs of enantiomers. Enantiomers have identical physical properties but differ in their interaction with plane-polarized light.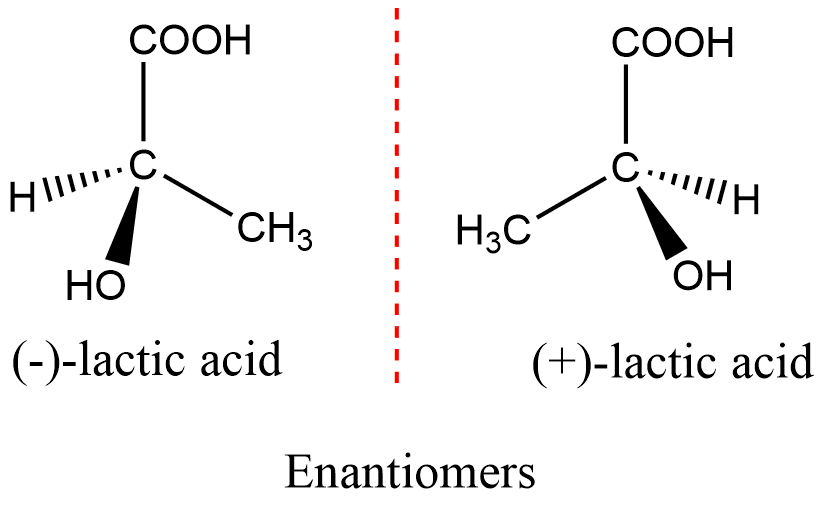
Preparation of Hydrocarbons
(i) Alkanes
1. Decarboxylation
Decarboxylation refers to the process of removal of CO2 from the molecules having -COOH group. Saturated monocarboxylic acid salt of sodium potassium on dry distillation with soda lime gives alkane.
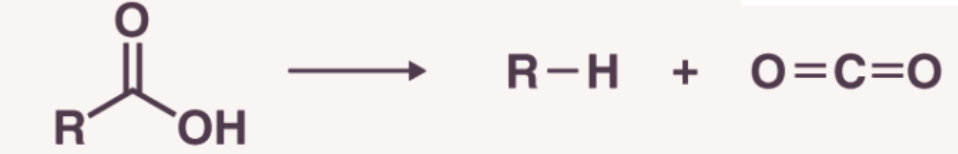
The alkane formed by decarboxylation process always contains one carbon atom less than the original acid. The yield is good in case of lower members but poor for higher members.
Soda lime is prepared by soaking quick lime CaO in caustic soda solution and then drying the products. It is generally written as NaOH + CaO. Its reaction is milder than caustic soda. Otherwise the reaction will occur violently. Also CaO used alongwith NaOH keeps it dry (NaOH is hygroscopic) to aid fusion.
The decarboxylation of sodium formate yields H2.
2. Wurtz Reaction
In Wurtz reaction a solution of alkyl halide in ether on heating with sodium gives alkane.

| An alkyl halide on Wurtz reaction leads to the formation of symmetrical alkane having an even number of carbon atoms. Two different alkyl halides, on Wurtz reaction give all possible alkanes. |
The different involved are steps are:
- CH3X + 2Na + C2H5X → CH3CH2CH3 + 2NaX
- CH3X + 2Na + C2H5X →CH3CH3 + 2NaX
- C2H5X + 2Na + C2H5X → C2H5C2H5 + 2NaX
The separation of mixture into individual members is not easy because their boiling points are near to each other and thus Wurtz reaction is not suitable for the synthesis of alkanes containing odd number of carbon atoms.
When Zn is used in Wurtz reaction in place of Na, the reaction is named as Frankland method.
Limitations of Wurtz reaction :
- Methane can not be obtained by this method
- The reaction fails in case of tertiary halides
Mechanism of Wurtz reaction :
The mechanism of Wurtz reaction is although not clear however two mechanisms are proposed for this reaction.
The first proposed mechanism of Wurtz reaction involved formation of an Intermediate organometallic compound:
RX + 2Na → [RNa] + NaX (Intermediate)
RX + [RNa] → R-R + NaX
Another proposed mechanizm of Wurtz reaction involved formation of Intermediate free radicals:
- RX + Na → R. (Free Radicals)+ Nax
- R. + R. → R-R
3. By the Reduction of Alkyl Halides
Alkyl halides on reduction with nascent hydrogen form alkanes. R-X + 2[H]→R-H + HX. The nascent hydrogen may be obtained by any one of the following
Alkyl halides can also be reduced catalytically to alkane by H2/Pd or LiAIH4 or by H2/Ni. The yields are generally high and the hydrocarbons formed are pure.
Note : Zn-Cu couple is prepared by adding Zn granules in aqueous CuSO4 solution where copper is deposited on the Zn pieces.
4. By Hydrogenation of Alkenes((>C=C<) : Sabatier and Senderen's Method
Alkenes and alkynes on catalytic hydrogenation give alkanes
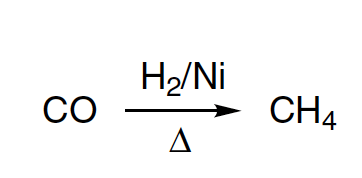
Catalyst Ni is used in finely divided form. If Pt or Pd are used as catalyst, reaction occurs at normal temperature. Also some times Raney nickel is used as catalyst. It is obtained by boiling Ni-AI alloy with NaOH, when AI dissolves leaving Ni in finely divided state. The filtered, washed and died Ni is known as Raney Nickel. Raney Ni is effective at room temperature and atmospheric pressure.
5. Kolbe's Electrolysis Method
Alkanes are formed, on electrolysis of concentrated aqueous solution of sodium or potassium salt of saturated mono carboxylic acids
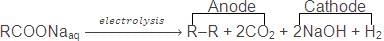
Electrolysis of an acid salt gives symmetrical alkane. However, in case of mixture of carboxylic acid salts, all probable alkanes are formed.

6. By Grignard Reagents
Organic compounds in which a metal atom is directly linked to carbon atom are known as organometallic compound.
Examples include: HC≡CNa, (C2H5)4 Pb, (C2H5)2 Zn
Alkyl or aryl magnesium halide (R-MgX) are also called Grignard reagents or organometallic compounds.Grignard reagent on double decomposition with water or with other compounds having active H(the hydrogen attached on O, N, F or triple bonded carbon atom are known as active hydrogen) give alkane.
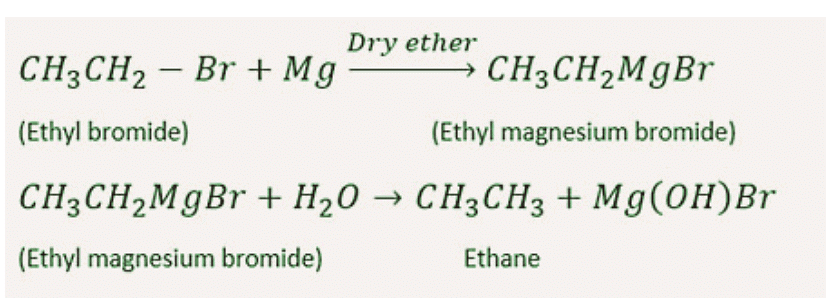
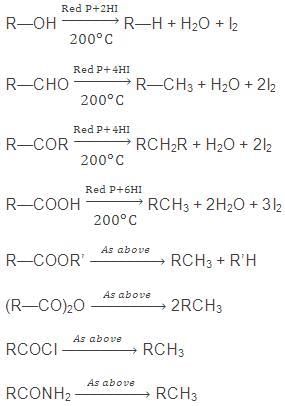
7. By Reduction of Alcohols, Aldehydes, Ketones or Fatty Acids and their Derivatives
The reduction of either of the above in presence of red P & HI gives corresponding alkane.
8.By Reduction of Carbonyl Compounds
The reduction of carbonyl compounds by amalgamated zinc and conc. HCI also yields alkanes. This is Clemmensen reduction.
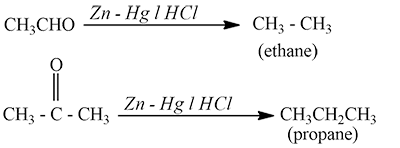
Carbonyl compounds may also be reduced to alkanes by Wolf Kishner reaction
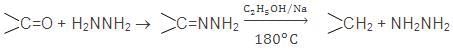
9.By the Hydrolysis of AI or Be Carbides
Only CH4 can be obtained by the hydrolysis of Be or Al carbides.
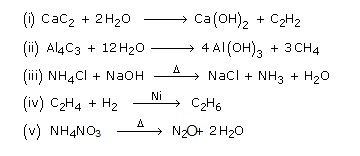
Note :
- Calcium carbide reacts with water to give acetylene.
- Magnesium carbide, Mg2C2 reacts with water to give propyne.
- CH4 can be obtained by passing a mixture of H2S and CS2 through red and Cu tube
10.By Hydroboration of Alkenes
Alkenes on hydroboration give trialkyl borane as a result of addition of diborane on olefinic bond. This trialkyl borane on treatment with acetic acid or propanoic acid yields alkane.
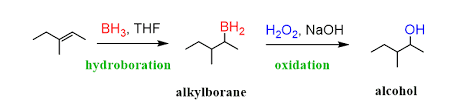
11. By Corey- House Synthesis
Alkyl chloride say chloroethane reacts with lithium in presence of ether to give lithium alkyl then reacts with CuI to give lithium dialkyl cuprate. This lithium dialkyl cuprate now again reacts with alkyl chloride to given alkane.
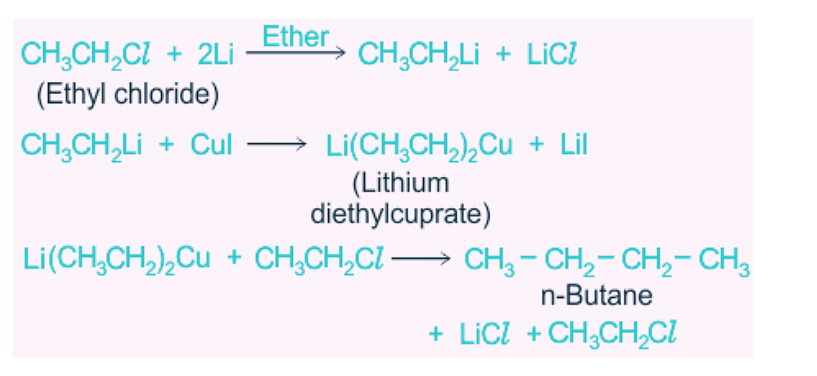
Li(CH3CH2)2Cu + CH3CH2CI → CH3CH2CH2CH3 + CH3CH2Cu + LiCl
(ii) Alkenes
General formula: CnH2n
Most of the reactions involving the preparation of alkenes involve the elimination process. There are 3 mechanisms suggested for the elimination reactions. All these eliminations are β- eliminations.
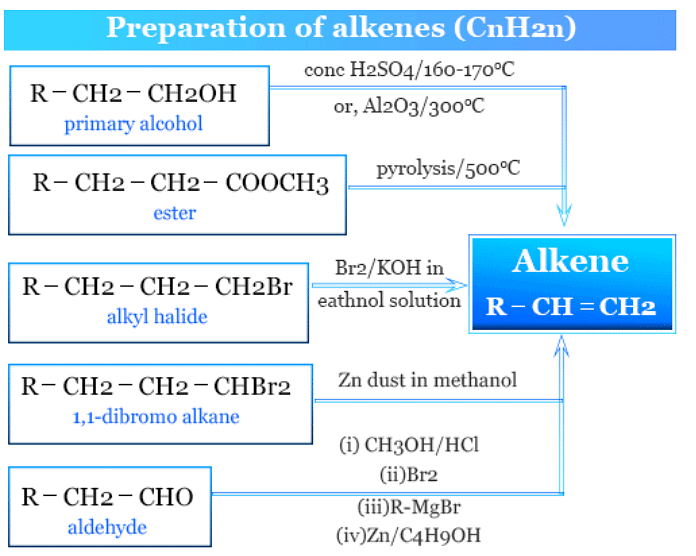
1. Elimination reactions (E2 and E1 mechanisms):
- Alkenes can be prepared through elimination reactions that involve the removal of a leaving group from an adjacent carbon.
- Example: Dehydration of ethanol (C2H5OH) to ethene (C2H4) through an E1 mechanism using concentrated sulfuric acid (H2SO4):
C2H5OH → C2H4 + H2O
2. Hydration reactions:
- Alkenes can undergo hydration reactions, adding water to the double bond.
- Example: Hydration of propene (CH3CH=CH2) to form 2-propanol (CH3CHOHCH3) using sulfuric acid (H2SO4) as a catalyst:
CH3CH=CH2 + H2O → CH3CHOHCH3
3. Oxidation reactions:
- Alkenes can be oxidized using various reagents to yield different products.
- Example: Oxidation of ethene (C2H4) with hot potassium permanganate (KMnO4) to produce ethylene glycol (HOCH2CH2OH):
C2H4 + 2 KMnO4 → HOCH2CH2OH + 2 KOH + 2 MnO2
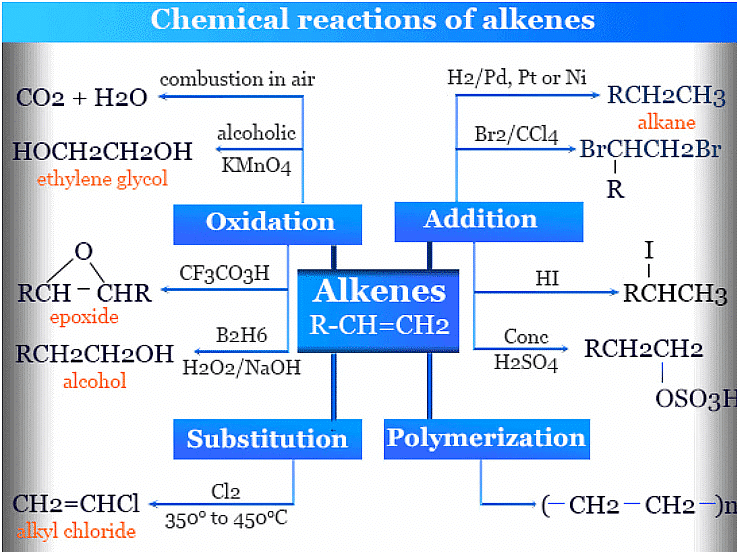
(iii) Alkynes
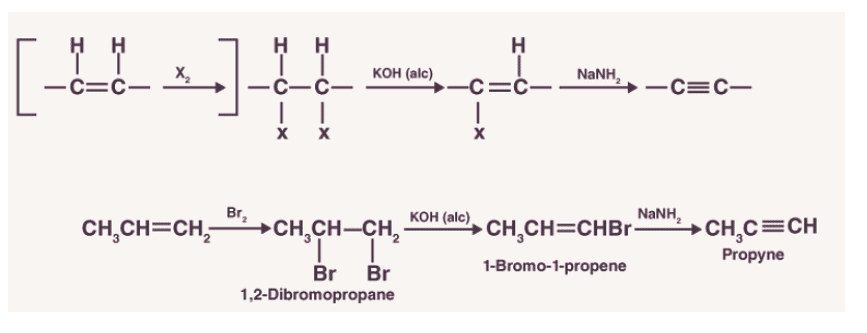
1.Dehydrohalogenation of alkyl dihalides
In this method of preparation, alkenes are made to react with a halogen. Due to this reaction, a substituted alkane is obtained. Alkanes formed are further passed through alcoholic KOH in order to form substituted alkenes. It is then made to react with sodium amide to form alkynes. This process is called dehydrohalogenation as hydrogen is eliminated along with a halogen in order to obtain an alkyne.
2. Preparation of acetylene from calcium carbide.
For large-scale production of an alkyne, calcium carbide (CaC2) is made to react with water. It is prepared by heating quicklime in the presence of coke. Quicklime is obtained by introducing limestone to heat.
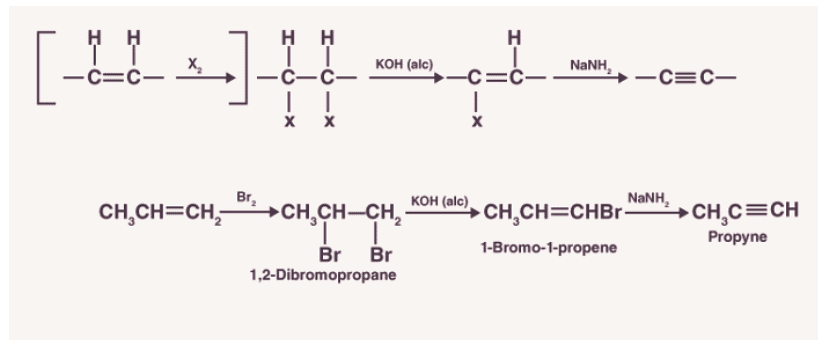
The reactions for the preparation of acetylene from calcium carbide are as shown below:
3.Preparation from vicinal dihalides
- Dihalides are obtained from corresponding alkenes by the addition of halogens (Group 17 elements).
- Alkynes are obtained from vicinal dihalides by dehydrohalogenation which is carried out in two steps:The first step is to prepare the unsaturated halides. The halides formed have a halogen attached to a double-bonded carbon.
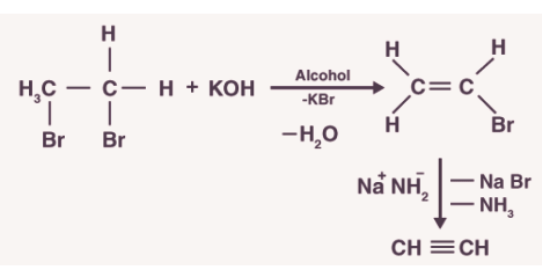
- These halides are called vinylic halides which are not reactive in nature. These halides are made to react with the strong base which results in the formation of alkynes.
- Metal acetylides are used to convert small alkynes into large ones.
(iv) Benzene
1. From ethyne (acetylene):
- Benzene can be synthesized from ethyne (acetylene) through a series of reactions, including partial hydrogenation and subsequent dehydrogenation.
- Example: Conversion of ethyne (C2H2) to benzene (C6H6) through partial hydrogenation and dehydrogenation reactions using catalysts like palladium (Pd) or platinum (Pt):
3 C2H2 → C6H6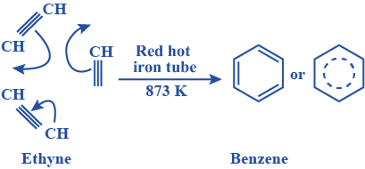
2. From phenol:
- Benzene can be prepared from phenol through various methods, including the Dow process, which involves the decarboxylation of sodium phenoxide.
- Example: Decarboxylation of sodium phenoxide (C6H5ONa) to produce benzene (C6H6) using soda-lime: C6H5ONa → C6H6 + Na2CO3

3. From aniline:
- Benzene can also be synthesized from aniline, an amine compound, through certain chemical reactions, such as the Hofmann rearrangement.
- Example: Conversion of aniline (C6H5NH2) to benzene (C6H6) through the Hofmann rearrangement using chlorine (Cl2) and sodium hydroxide (NaOH):
C6H5NH2 + Cl2 + 2 NaOH → C6H6 + NaCl + NaOCl + 2 H2O
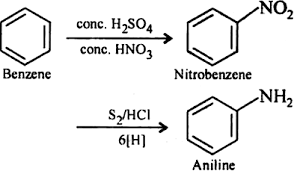
These are some of the common methods for the preparation of alkynes and benzene. The choice of method depends on the starting materials and desired products.
Uses of Hydrocarbons
Hydrocarbons have numerous uses across various industries due to their diverse properties.
Here are some common applications of hydrocarbons:
- Fuel and Energy Production: Hydrocarbons are the primary components of fossil fuels such as gasoline, diesel, and natural gas. These fuels are used for transportation, power generation, heating, and cooking. Hydrocarbons also serve as feedstocks for the production of alternative fuels like biofuels and hydrogen.
- Chemical Manufacturing: Hydrocarbons are essential in the production of a wide range of chemicals. They serve as raw materials or precursors for the synthesis of plastics, solvents, detergents, fertilizers, pharmaceuticals, synthetic fibers, rubber, and many other chemical products.
- Lubricants and Greases: Certain hydrocarbons, such as mineral oils and synthetic hydrocarbon-based fluids, are used as lubricants and greases. They reduce friction and provide protection to moving parts in machinery and engines, enhancing their performance and longevity.
- Petrochemical Industry: Hydrocarbons derived from petroleum, such as ethylene and propylene, are the building blocks for the petrochemical industry. These compounds are used as feedstocks to produce various chemicals, including plastics, resins, adhesives, synthetic fibers, and rubber.
- Heat Transfer Fluids: Hydrocarbon-based fluids with high thermal stability, such as heat transfer oils, are used in industrial processes to transfer heat between equipment and systems. These fluids help maintain temperature control in applications such as HVAC systems, chemical reactors, and heat exchangers.
|
114 videos|263 docs|74 tests
|
FAQs on Hydrocarbons: Classification, Properties, Preparation & Uses - Chemistry Class 11 - NEET
| 1. What are hydrocarbons? |  |
| 2. What are the different classifications of hydrocarbons? |  |
| 3. What are the physical properties of hydrocarbons? |  |
| 4. What are the chemical properties of hydrocarbons? |  |
| 5. How are hydrocarbons prepared? |  |
| 6. What are the uses of hydrocarbons? |  |






















