Hydrogen Bonding | Chemistry Class 11 - NEET PDF Download
Hydrogen Bonding
- When a hydrogen atom linked to a highly electronegative atom (like F, O, or N) comes under the influence of another strongly electronegative atom, then a weak bond is developed between them which is called a hydrogen bond.
- It is represented by the dotted line as follows:
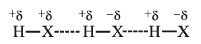
- As a result of hydrogen bonding, the H–atom links the two electronegative atoms simultaneously, one by a covalent bond and the other by a hydrogen bond. Hence it is said to form a hydrogen bridge.
- It is merely a strong electrostatic attractive force and not a normal chemical bond. It is very weak (strength about 2-10 Kcal/mol).
Conditions for Hydrogen bonding
(a) The molecule must contain a highly electronegative atom linked to H-atom. The higher the electronegativity, the more is the polarization of the molecule.
(b) The size of the electronegative atom should be small. The smaller the size the greater is the electrostatic attraction.
Types of Hydrogen bonding
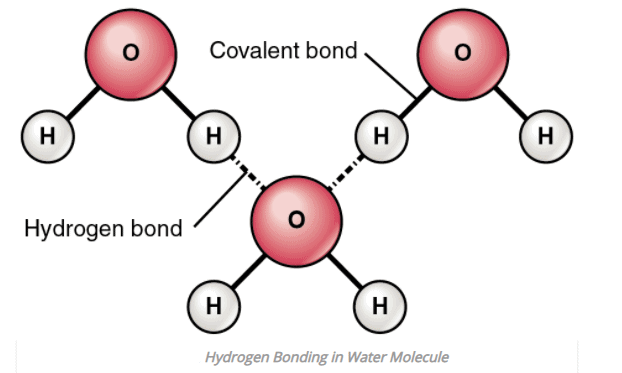
1. Intermolecular Hydrogen bonding
- When hydrogen bonding takes place between different molecules of the same or different compounds, it is called intermolecular hydrogen bonding.
- Example: HF, H2O, ROH (same compound) water-alcohol, water ammonia (different compound) etc.
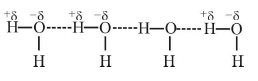
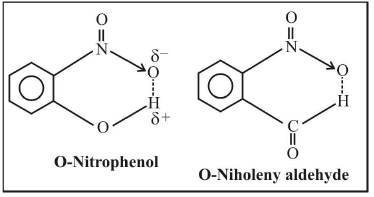
2. Intramolecular hydrogen bonding
- The hydrogen bonding takes place within a molecule itself.
- It takes place in compounds containing two groups such that one group contain the H-atom linked to an electronegative atom and the other group contains a highly electronegative atom linked to a lesser electronegative atom.
- The bond is formed between the H-atom of one group with the more electronegative atom of the other group.
- Example: O-Nitrophenol, O-Niholeny aldehyde
 Vander Waal’s Forces
Vander Waal’s Forces
- This type of attractive forces occurs in the case of non-polar molecules such as H2, O2, Cl2, CH4, CO2, etc.
- The existence of weak attractive forces among the nonpolar molecule was first proposed by Dutch scientist J.D. Vander Waal.
- Vander Waal force ∝ molecular weight ∝ Boiling point
Types of Vander Waal’s force
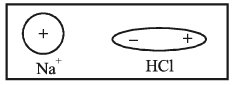
1. Ion-Dipole attraction: This force is between an ion such as Na+ and a polar molecule such as HCl.
2. Dipole-Dipole attraction: It is again in between two polar molecules such as HF and HCl.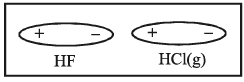
3. Ion-Induced dipole attraction: In this case, a neutral molecule is induced by an ion as a dipole.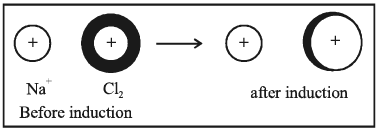
4. Dipole-induced dipole attraction - In this case, a neutral molecule is induced as a dipole by another dipole.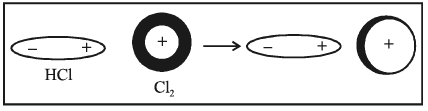
5. Induced dipole-induced dipole attraction or London dispersion force between two non-polar molecules as in Cl2, He etc.
Note:
The relative strength of various bonds is as follows:
Ionic bond > Covalent bond > Metallic bond > H-bond > Vander waal bond.
Metallic Bond
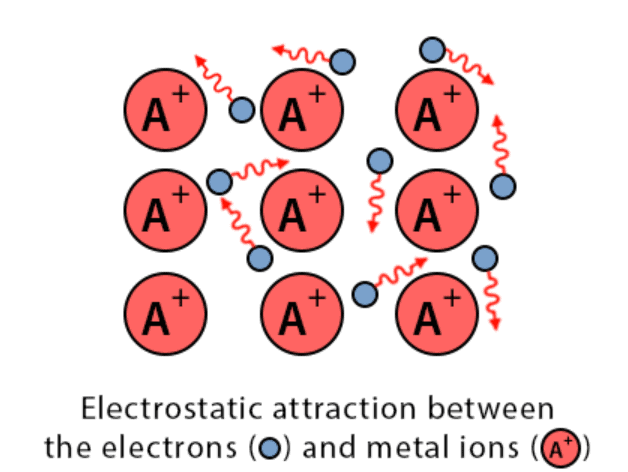
- The constituent particles of metallic solids are metal atoms that are held together by the metallic bond.
- A metal atom is supposed to consist of two parts, valence electrons and the remaining part (the nucleus and the inner shells) called the kernel.
 Metallic Bond
Metallic Bond
- The kernels of metal atoms occupy the lattice sites while the space between the kernel is occupied by valence electrons.
- Due to small ionisation energy, the valence electrons of metal atoms are not held by the nucleus firmly. Therefore, the electrons leave the field of influence of one kernel and come under the influence of another kernel.
- Thus the electrons are not localised but are mobile. The simultaneous attraction between the kernels and the mobile electrons which hold the kernel together is known as a metallic bond. This model is known electron sea model.
|
114 videos|263 docs|74 tests
|
FAQs on Hydrogen Bonding - Chemistry Class 11 - NEET
| 1. What is hydrogen bonding? |  |
| 2. How does hydrogen bonding affect the physical properties of substances? |  |
| 3. What is Vander Waal's force? |  |
| 4. How do Vander Waal's forces compare to hydrogen bonding in terms of strength? |  |
| 5. What is metallic bonding? |  |
















