Importance & Scope of Chemistry | Chemistry Class 11 - NEET PDF Download
"Chemistry is the science of molecules and their transformations. It is the science not so much of the one hundred elements but of the infinite variety of molecules that may be built from them.
- Roald Hoffmann"
What is Chemistry?
Chemistry is the scientific study of matter, its properties, and its behavior, as well as the changes that it undergoes during chemical reactions. Matter refers to anything that has mass and takes up space, including solids, liquids, gases, and even plasma.

- In chemistry, scientists investigate the properties of matter, such as its composition, structure, and interactions with other substances. They also study how matter can be transformed through chemical reactions, which involve breaking and forming chemical bonds between atoms and molecules.
Overall, chemistry is a crucial scientific field that helps us better understand the natural world and develop new technologies and materials that benefit society.
What are the Branches of Chemistry?
Chemistry is a broad field that encompasses many different branches, each with its own focus and area of study. Here are some of the major branches of chemistry:
- Organic Chemistry: Organic chemistry is the study of the structure, properties, composition, reactions, and preparation of Carbon-containing compounds. These compounds called ”Hydrocarbons” contain carbon along with Hydrogen and may also include elements like oxygen, halogens, sulfur, nitrogen, and phosphorus.
- Inorganic Chemistry: This branch of chemistry deals with the study of compounds of all other elements except carbon, which include metals(other than carbon), minerals, and organometallic compounds.
- Physical Chemistry: The explanation of fundamental principles governing various chemical phenomena is the main concern of this branch. It deals with the principles of physics involved in chemical interactions.
- Industrial Chemistry: The chemistry involved in industrial processes is studied under this branch.
- Analytical Chemistry: This branch deals with the qualitative and quantitative analysis of matter. It deals with obtaining, processing, and communicating information about the composition and structure of matter.
- Biochemistry: This branch deals with the study of chemical processes occurring in living matter.

What all will you study in Class 11 & Class 12 Chemistry?
In Class 11-12 Chemistry, you will study a wide range of topics that build upon the foundational knowledge gained in earlier grades. Here is an overview of the key areas you will explore:
Class 11 Chemistry:
- Some Basic Concepts of Chemistry: This chapter introduces fundamental concepts such as the mole concept, chemical equations, and stoichiometry.
- Structure of Atom: You will learn about atomic structure, electron configurations, and the organization of subatomic particles within an atom.
- Classification of Elements and Periodicity in Properties: This chapter focuses on the periodic table and the classification of elements based on their properties and trends.
- Chemical Bonding and Molecular Structure: You will study different types of chemical bonds and their role in forming molecules and compounds.
- Thermodynamics: You will delve into the study of energy changes in chemical reactions, including concepts like heat, work, and enthalpy.
- Equilibrium: This topic covers chemical equilibrium, reversible reactions, and factors affecting the equilibrium state.
- Redox Reactions: You will learn about oxidation-reduction reactions, electron transfer, and balancing redox equations.
- Organic Chemistry - Some Basic Principles and Techniques: You will delve into the basics of organic chemistry, including nomenclature, functional groups, and purification techniques.
- Hydrocarbons: This topic covers the different types of hydrocarbons, such as alkanes, alkenes, and alkynes, and their reactions.

Class 12 Chemistry:
- Solutions: This chapter focuses on the properties of solutions, factors affecting solubility, and different types of solutions.
- Electrochemistry: You will learn about redox reactions, electrochemical cells, electrolysis, and applications of electrochemistry in batteries and corrosion prevention.
- Chemical Kinetics: This chapter covers the rates of chemical reactions, factors influencing reaction rates, and the study of reaction mechanisms.
- The d & f Block Elements: You will study the properties, characteristics, and reactions of transition metals (d-block) and inner transition metals (f-block).
- Coordination Compounds: This topic delves into the chemistry of coordination compounds, including complex ions, ligands, and their bonding.
- Haloalkanes and Haloarenes: You will explore the nomenclature, properties, and reactions of halogenated hydrocarbons.
- Alcohols, Phenols, and Ethers: This chapter focuses on the properties, preparation methods, and reactions of alcohols, phenols, and ethers.
- Aldehydes, Ketones, and Carboxylic Acids: You will study the properties, synthesis, and reactions of aldehydes, ketones, and carboxylic acids.
- Amines: This topic covers the properties, classification, and reactions of amines, including their synthesis and applications.
- Biomolecules: You will explore the different types of biomolecules, including carbohydrates, proteins, nucleic acids, and lipids, as well as their structure

What is the Importance & Scope of Chemistry?
Chemistry is a fundamental science that is concerned with the composition, properties, and behavior of matter. It is an essential field of study with a wide range of applications in almost every aspect of our daily lives. Here are some of the key reasons why chemistry is important and the scope of the field:
 Importance of Chemistry
Importance of Chemistry
There are many instances in your day-to-day life that involve chemistry, its applications, and its principles. Let us look at them one by one.
1. Chemistry in Supply of Food
- The chemistry study provided the world with chemical fertilizers such as urea, calcium superphosphate, sodium nitrate, and ammonium sulphate. These chemicals have helped greatly in increasing the yield of fruits, vegetables, and other crops. Thus, we can cater to the ever-growing demand for food.
- It has helped protect crops from insects and harmful bacteria by using certain effective insecticides, fungicides, and pesticides.
- Chemistry also led to the discovery of preservatives. These chemicals have greatly helped to preserve food products for a longer period. It has given methods to test the presence of adulterants. This ensures the supply of pure foodstuff which further enhances the quality of life.

2. Chemistry's Contribution in Improved Health & Sanitation
- Chemistry provided humanity with a large number of life-saving drugs. We could find a cure for dysentery and pneumonia due to the discovery of sulphur drugs and penicillin.
- Besides this, life-saving drugs like cisplatin and taxol are effective for cancer therapy, and AZT (Azidothymidine) is used for people with AIDS.
- Some of the common drugs that chemistry has blessed us with include:
(i) Analgesics: To reduce the pain of different types.
(ii) Antibiotics: To curb infection and cure diseases.
(iii) Tranquillizers: To reduce stress and bring about calm and peace to patients suffering from mental diseases.
(iv) Antiseptics: To stop infection of the wounds.
(v) Disinfectants: To kill the microorganism present in toilets, floors, and drains.
(vi) Anesthetics: Discovery of anesthetics has made surgical operations more successful and less painful.
(vii) The use of insecticides such as DDT and Gammexane has greatly reduced the dangers of diseases caused by rats, mosquitoes, and flies.

3. Scope of Chemistry in Saving the Environment
4. Chemistry increasing Comfort, Pleasure & Luxuries
Because of the advancements in science and chemistry discoveries, we lead a more comfortable life today. You may ask how? Let us see below.
- Synthetic Fibers: These are more attractive, comfortable, and durable. They include terylene, nylon, and rayon. They are easy to wash, dry quickly and do not need ironing. Chemistry provides a large number of synthetic dyes that imparts bright and fast color to clothes.
Types of synthetic fibers
- Building Materials: By supplying steel and cement, chemistry helps construct safer homes and multi-storage buildings. It also helps in the construction of long-lasting and durable dams and bridges.
- Supply of Metals: Metals like gold, silver, copper, iron, aluminum, zinc and a large number of alloys are used for making various objects. These include ornaments, utensils, coins and many industrial and agricultural implements.
- Articles of domestic use: Chemistry has made our homes more comfortable by supplying many articles on domestic uses.
Example: It includes detergents, oils and fats, sugar, paper, glass, plastic, paints, cosmetics, perfumes, cooking gas, etc. We can beat the heat in summer by using refrigerants like ammonia, liquid sulphur dioxide, and freon.
 Articles of Domestic Use
Articles of Domestic Use
- Entertainment: Cinema, video cameras, and simple cameras make use of films made of Celluloid and coated with suitable chemicals. Fireworks that amuse us at festivals and marriages are chemical products. Can you imagine how boring life would have been if you wouldn’t have been able to take all those cute selfies? Also, much before we had smartphones in our hands, we used film cameras which made use of silver bromide( a chemical) for developing a photo from its photographic film. Interesting isn't it?
5. Chemistry in Transport and Communication
All the different means of transportation including cars, aircraft, and trains, are powered by products of fractional distillation of crude oil. Fractional distillation is a process that makes use of applications of chemistry to break different hydrocarbons into fractions at different boiling points.6. Chemistry creating Nuclear Atomic Energy
- Given the current scenario, when exhaustible natural resources like coal and petroleum need to be saved, Chemistry has come to the rescue by providing an alternative source of energy which is nuclear energy.
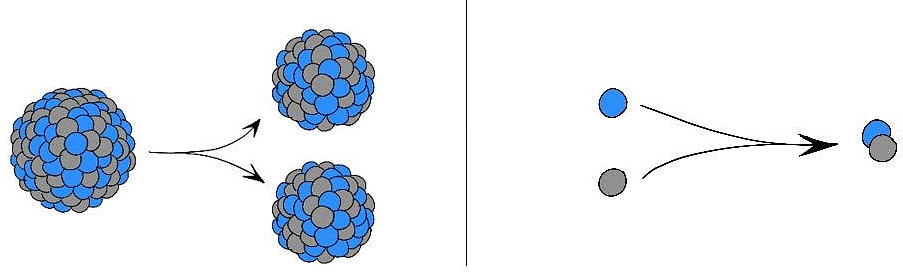 Nuclear Fission (Left); Nuclear Fusion (Right)
Nuclear Fission (Left); Nuclear Fusion (Right)
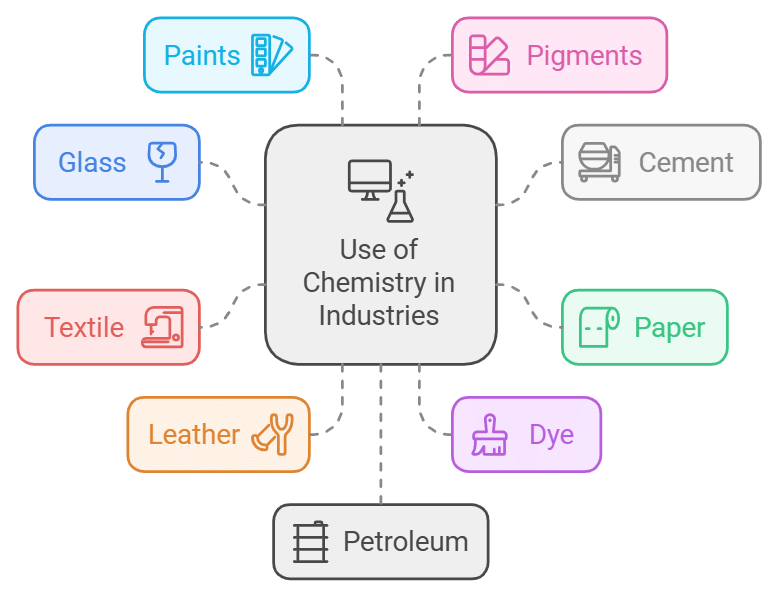
7. Scope of Chemistry in Industries
- Chemistry plays an important and useful role in the development and growth of several industries. This includes industries like glass, cement, paper, textile, leather, dye, etc.
- We also see huge chemistry applications in industries like paints, pigments, petroleum, sugar, plastics, and Pharmaceuticals. It has also helped increase sulphuric acid, nitric acid, Ammonia, and hydrogenated oils by providing suitable catalysts.

8. Scope of Chemistry in War
- Chemical warfare is the use of toxic properties of chemical substances to kill, injure and incapacitate the enemy in war.
- Chemistry plays an important role in discovering highly explosive substances such as TNT, nitroglycerine, and dynamite.
- The cities of Hiroshima and Nagasaki experienced the biggest atomic bombings in world war 2 killing lakhs of people
 The destruction caused by Atom Bomb
The destruction caused by Atom Bomb
9. Chemistry of Cosmetics in Everyday Life
Cosmetics contain the following categories of chemicals.
- Talc: The chemical formulation in talc is hydrated magnesium silicate. Talk is a major ingredient in face powders, concealers, etc.
- Alcohol: Alcohols are major ingredients in perfumes.
- Formaldehyde: Formaldehyde is a very common component in many cosmetic items such as nail paint remover, shampoos, conditioners, shower gels, creams, toothpaste etc.
- Emulsifiers: These increase the stability of the emulsion. For example, Potassium cetyl sulfate.
- Preservatives: These are added to cosmetics to increase their shelf life—for example, benzyl alcohol, and salicylic acid.
- Thickeners: These give an appealing consistency. For example, Cetyl alcohol, Stearic acid.
- Emollients: These soften the skin by preventing water loss. For example, Glycerine, and zinc oxide.
- Glimmer and Shiners: For example, mica, bismuth oxychloride.

10. Other Examples of Chemistry in Everyday Life
- The Expiration Date on Bottled Drinking Water:
Have you ever wondered why there is an expiration date on a bottle of drinking water? After all, it is just water, isn’t it? Well, most of us haven’t even noticed that there is, in fact, an expiration date on that bottle. The idea behind instilling an expiration on bottled drinking water is to standardize the packaging quality. The actual expiration date signifies if the expiration date is up, the taste of the water will be different as there is a chance of the chemicals in the packaging material ruining the quality of the water. - Elements in the Human Body:
We all know our body is about 60% water, but what composes its rest? Carbon, Hydrogen, Nitrogen, and Oxygen. These elements compose 96% of the human body. At the same time, the rest, 4%, is composed of about 60 elements. Some of these elements include calcium, phosphorus, potassium, and sulphur. - Sunblock and Sunscreen:
There are two kinds of rays from the sun that are particularly bad for us, UV-A & UV-B. Sunscreen’s action is, as the name, suggests it functions as a screen and offers protection from sunburns which is caused by UV-B. At the same time, sunblock has a more of reflective nature and blocks both UV-A & UV-B radiations.
Frequently Asked Questions
1. What are the main branches of chemistry?
Chemistry is the science of matter properties. All matter is composed of atoms and molecules. There are 5 main branches of chemistry:
- Organic chemistry
- Inorganic chemistry
- Physical chemistry
- Biochemistry
- Analytical Chemistry
Organic chemistry is simply the study of carbon and its compounds mainly with hydrogen. oxygen, sulphur, nitrogen, and halogens. Organic chemistry initially involves the study of compounds that could only be obtained from living organisms. Hence the word “ORGANIC” Approximately 7 million different organic compounds are known and present, while there are only 1.5 million known inorganic compounds. This large number of organic compounds arise from the unique property of carbon called catenation, which means self-linking.
3. List down some problems caused by Chemistry.- Nuclear energy is useful, but the disposal of nuclear waste poses a serious problem to humanity. Also, nuclear disasters are a constant threat to humanity.
- Phonograph records have added to our pleasure in listening to music, but they are made of polyvinyl chloride. This is produced from vinyl chloride, which can cause liver cancer in industrial workers.
- Pharmaceutical drugs have helped in eliminating infectious diseases, but their overuse can be very harmful. Advancement in Chemistry has given drugs like LSD, cocaine, and methamphetamine. These prove to be a curse to society.
|
129 videos|233 docs|88 tests
|
FAQs on Importance & Scope of Chemistry - Chemistry Class 11 - NEET
| 1. What is the definition of Chemistry? |  |
| 2. What are the main branches of Chemistry? |  |
| 3. What topics are covered in Class 11 and Class 12 Chemistry? |  |
| 4. Why is Chemistry considered important? |  |
| 5. What is the scope of Chemistry in career opportunities? |  |

|
Explore Courses for NEET exam
|

|