Important p-Block Elements & Their Compounds Formulas for JEE and NEET
TRENDS IN PROPERTIES OF p-BLOCK ELEMENTS.
(A) GROUP 13 ELEMENTS : THE BORON FAMILY
Oxidation state and trends in chemical reactivity :
General Oxidation State = + 3.
Reactivity towards acids and alkalies
Reactivity towards halogens
2E(s) + 3X2(g) → 2EX3 (s) (X = F, Cl Br, I)
BORON (B):
Some Important Reactions of Boron and its compounds :


- Small amines such as NH3, CH3NH2 and (CH3)2NH give unsymmetrical cleavage of diborane.

- Large amines such as (CH3)3N and pyridine give symmetrical cleavage of diborane.


(B) GROUP 14 ELEMENTS : THE CARBON FAMILY
Carbon (C), silicon (Si), germanium (Ge), tin (Sn) and lead (Pb) are the members of group 14.
Electronic Configuration = ns2np2.
Oxidation states and trends in chemical reactivity
Common oxidation states = +4 and +2. Carbon also exhibits negative oxidation states. In heavier members the tendency to show +2 oxidation state increases in the sequence Ge < Sn < Pb.
(i) Reactivity towards oxygen : All members when heated in oxygen form oxides. There are mainly two types of oxides, i.e. monoxide and dioxide of formula MO and MO2 respectively.
(ii) Reactivity towards water : Tin decomposes steam to form dioxide and dihydrogen gas.
(iii) Reactivity towards halogen : These elements can form halides of formula MX2 and MX4 (where X = F, Cl Br, I). Stability of dihalides increases down the group.
ANOMALOUS BEHAVIOUR OF CARBON :
Catenation : The order of catenation is C > > Si > Ge = Sn. Lead does not show catenation. Due to the property of catenation and pπ-pπ bonds formation, carbon is able to show allotropic forms.

Allotropes of Carbon Diamond: Crystalline lattice sp3 hybridisation and linked to four other carbon atoms by using hybridised orbitals in tetrahedral manner. The C-C bond length is 154 pm. and produces a rigid three dimensional network of carbon atoms.
Graphite : Graphite has layered structure. Layers are held by van der Waal’s forces and distance between two layers is 340 pm. Each layer is composed o f planar hexagonal rings of carbon atoms. C - C bond length within the layer is 141.5 pm. Each carbon atom in hexagonal ring undergoes sp2 hybridisation graphite conducts electricity along the sheet. Graphite cleaves easily between the layers and therefore, it is very soft and slippery. For this reason graphite is used as a dry lubricant in machines running at high temperature.
Fullerenes : C60 molecule has a shape like soccer ball and called Buckminsterfullerene. It contains twenty six -membered rings and twelve five membered rings. This ball shaped molecule has 60 vertices and each one is occupied by one carbon atom and it also contains both single and double bonds with C - C distance of 143.5 pm and 138.3 pm respectively.
SOME IMPORTANT REACTIONS OF CO, CO2 AND METAL CARBIDES :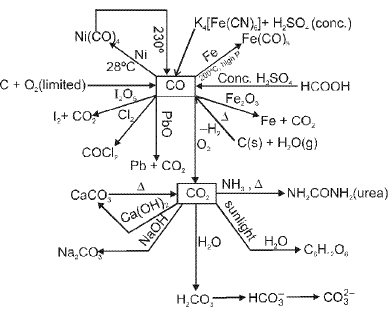

- CLASSIFICATION OF SILICATES :
(A) Orthosilicates:
(B) Pyrosilicate:
(C) Cyclic silicates :

(D) Chain silicates :

(E) Two dimensional sheet silicates : In such silicates, three oxygen atoms of each tetrahedral are shared with adjacent SiO4-4 tetrahedrals. Such sharing forms two dimension sheet structure with general formula (Si2O5)n2n-
(F) Three dimenstional sheet silicates : These silicates involve all four oxygen atom in sharing with adjacent SiO4-4 tetrahedral units. - SILICONES :
- Silicones can be prepared from the following types of compounds only.
(i) R3SiCI (ii) R2SiCI2 (iii) RSiCI3 - Silicones from the hydrolysis of (CH3)3 SiCI

- Silicones from the hydrolysis of a mixture of (CH3)3 SiCI & (CH3)2 SiCI2

- When a compound like CH3SiCI3 undergoes hydrolysis, a complex cross-linked polymer is obtained.
- The hydrocarbon layer along the silicon-oxygen chain makes silicones water-repellent.
COMPOUNDS OF LEAD:

COMPOUNDS OF TIN :
(C) GROUP 15 ELEMENTS : THE NITROGEN FAMILY
Electronic Configuration : ns2 np3
Atomic and Ionic Radii : Covalent and ionic (in a particular state) radii increase in size down the group.
Physical Properties: All the elements of this group are polyatomic. Metallic character increases down the group. The boiling points , in general, increase from top to bottom in the group but the melting point increases upto arsenic and then decreases upto bismuth. Except nitrogen , all the elements show allotropy.
Chemical Properties :
Oxidation States and trends in a chemical reactivity :
The common oxidation states of these elements are -3, +3 and +5. The stability of +5 oxidation state decreases and that of +3 state increases (due to inert pair effect) down the group ; Bi3+ > Sb3+ > As3+; Bi5+ < Sb5+ < As5+ Nitrogen exhibits +1, +2, +4 oxidation states also when it reacts with oxygen.
Anomalous properties of nitrogen :
(i) The stability of hydrides decreases from NH3 to BiH3 which can be observed from their bond dissociation enthalpy. Consequently , the reducing character of the hydrides increases. Basicity also decreases in the order NH3 > PH3 > AsH3 > SbH3 > BiH3.
(ii) The oxide in the higher oxidation state of the element is more acidic than that of lower oxidation state. Their acidic character decreases down the group. The oxides of the type E2O3 of nitrogen and phosphorus are purely acidic, that of arsenic and antimony amphoteric and those of bismuth is predominantly basic.
(iii) Nitrogen does not form pentahalide due to non-availability of the d-orbitals in its valence shell. Pentahalides are more covalent than trihalides. Halides are hydrolysed in water forming oxyacids or oxychlorides.
(iv) These elements react with metals to form their binary compounds exhibiting -3 oxidation state , such as, Ca3N2 (calcium nitride) Ca3P2 (calcium phosphide) and Na3As2 (sodium arsenide).
NITROGEN (N) AND ITS COMPOUNDS :







Sn (a) dilute NH4NO3 4Sn+10HNO3 → 4Sn(NO3)2 + NH4NO3 + 3H2O
(b) concentrated NO2 Sn + 4 HNO3 → H2SnO3 + 4NO2 + H2O

PHOSPHORUS (P) AND ITS COMPOUNDS :
When white phosphorus is heated in the atmosphere of CO2 or coal gas at 573 K red phosphorus is produced, α-black phosphorus is formed when red phosphorus is heated in a sealed tube at 803 K. β-black phosphorus is prepared by heating white phosphorus at 473 K under high pressure.
Order of thermodynamic stability of various allotropes of phosphorus : black > red > white

Oxoacids of Phosphorus

(D) GROUP 16 ELEMENTS : THE OXYGEN FAMILY
Electronic Configuration : ns2np4.
Atomic and Ionic Radii : Due to increase in the number of shells , atomic and ionic radii increase from top to bottom in the group. The size of oxygen atoms is however, exceptionally small.
Physical Properties:
Oxygen and sulphur are non-metal, selenium and tellurium metalloids, whereas polonium is a metal. Polonium is radioactive and is short lived (Half-life 13.8 days). The melting and boiling points increase with an increase in atomic number down the group.
Catenation :
Tendency for catenation decreases down the group. This property is prominently displayed by sulphur (S8). The S—S bond is important in biological system and is found in some proteins and enzymes such as cysteine.
Chemical Properties
Oxidation states and trends in chemical reactivity : Elements of the group exhibit + 2, + 4, + 6 oxidation states but + 4 and + 6 are more common.
Anomalous behaviour of oxygen :
The anomalous behaviour of oxygen is due to its small size and high electronegativity. The absence of d orbitals in oxygen limits its covalency to four.
(i) Their acidic character increases from H2O to H2Te. The increase in acidic character can be understood in terms of decrease in bond (H-E) dissociation enthalpy down the group. Owing to the decrease in bond (H-E) dissociation enthalpy down the group, the thermal stability of hydrides also decreases from H2O to H2Po. All the hydrides except water possess reducing property and this property increases from H2S to H2Te.
(ii) Reducing property of dioxide decreases from SO2 to TeO2; SO2 is reducing while TeO2 is an oxidising agent. Oxides are generally acidic in nature.
(iii) The stabilities of the halides decrease in the order F > Cl > Br > I. Sulphur hexafluoride SF6 is exceptionally stable for steric reasons.
The well known monohalides are dimeric in nature, Examples are S2F2, S2CI2, S2Br2, Se2CI2 and Se2Br2. These dimeric halides undergo disproportionation as given below:
2Se2CI2 → SeCI4 + 3Se.
OXYGEN (O2) AND ITS COMPOUNDS :





(E) GROUP 17 ELEMENTS : THE HALOGEN FAMILY
Fluorine, chlorine, bromine, iodine and astatine are members of Group 17.
Electronic Configuration : ns2np5
Atomic and Ionic Radii
The halogens have the smallest atomic radii in their respective periods due to maximum effective nuclear charge.
Physical Properties
Fluorine and chlorine are gases, bromine is a liquid whereas iodine is a solid. Their melting and boiling points steadily increase with atomic number. The X-X bond disassociation enthalpies from chlorine onwards show the expected trend : Cl - Cl > Br - Br > F - F > I - I.
Chemical Properties
Oxidation states and trends in chemical reactivity
All the halogens exhibit -1 oxidation state. However, chlorine, bromine and iodine exhibit + 1, + 3, + 5 and + 7 oxidation states also.








(F) GROUP 18 ELEMENTS : (THE ZERO GROUP FAMILY)
helium, neon, argon, krypton , xenon and radon.
- Most abundant element in air is Ar. Order of abundance in the air is Ar > Ne > Kr > He > Xe.
Electronic Configuration : ns2np6
Atomic Radii Atomic radii increase down the group with increase in atomic number.
Physical properties All the noble gases are mono-atomic. They are colourless, and tasteless. They are sparingly soluble in water. They have very low melting and boiling points because the only type of interatomic interaction in these elements is weak dispersion forces,.
Chemical Properties : In general, noble gases are least reactive. Their inertness to chemical reactivity is attributed to the following reasons:
(i) The noble gases except helium (1s2) have completely filled ns2 np6 electronic configuration in their valence shell.
(ii) They have high ionisation enthalpy and more positive electron gain enthalpy.
The reactivity of noble gases has been investigated occasionally ever since their discovery, but all attempt to force them to react to form the compounds were unsuccessful for quite a few years. In March 1962, Neil Bartlett, then at the University of British Columbia, observed the reaction of a noble gas. First, he prepared a red compound which is formulated as O2+ PtF6-. He , then realised that the first ionisation enthalpy of molecular oxygen (1175 kJ mol-1) was almost identical with that xenon (1170 kJ mol-1). He made efforts to prepare same type of compound with Xe+ PtF6- by mixing Pt F6 and Xenon. After this discovery, a number of xenon compounds mainly with most electronegative elements like fluorine and oxygen, have been synthesised.
- If Helium is compressed and liquified it forms He(I) liquid at 4.2 K. This liquid is a normal liquid like any other liquid. But if it is further cooled then He(II) is obtained at 2.2 K, which is known as super fluid, because it is a liquid with properties of gases. It climbs through the walls of the container & comes out. It has very high thermal conductivity & very low viscosity.
CLATHERATE COMPOUNDS : During the formation of ice Xe atoms will be trapped in the cavities (or cages) formed by the water molecules in the crystal structure of ice. Compounds thus obtained are called clatherate compounds. Clathrate provides a convenient means of storing radioactive isotopes of Kr and Xe produced in nuclear reactors.



|
108 videos|286 docs|123 tests
|

|
Explore Courses for NEET exam
|

|




























