PH Scale: Indicators & Its Types | Science Class 10 PDF Download
| Table of contents |

|
| Indicators |

|
| Table: Indicator Change in Acidic Medium |

|
| Table: Indicator Change in Basic Medium |

|
| Some Solved Questions |

|
Indicators
The substances that show different colours in different mediums or the substances that are used to distinguish between an acid and base are called indicators.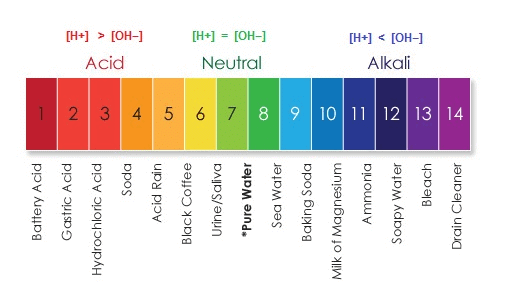 ph Scale
ph Scale
Types of Indicators
1. Natural Indicators: The indicators obtained from plants are called natural indicators.
(a) Litmus
- Litmus is a natural indicator which is extracted from “Lichens”, a plant belonging to thallophyta group. It is purple in colour. The pH range for litmus is 4.5 - 8.3 at room temperature.
- There are 2 types of litmus solution:
(i) Red Litmus: It is obtained by acidifying purple litmus extract
(ii) Blue Litmus: It is obtained by making purple litmus extract alkaline
In the laboratory litmus is available in the form of litmus paper; Red litmus and blue litmus paper. Red and Blue Litmus Paper
Red and Blue Litmus Paper
(b) Turmeric
- It is yellow in colour and turns red in the base and remains yellow in acids.
2. Synthetic Indicators: These indicators are manufactured in laboratories.
(a) Methyl Orange
- It is orange in colour and turns red in acids and yellow in the base.
(b) Phenolphthalein
- It is colourless and remains colourless in acids and turns pink in the base.
3. Olfactory Indicators: These indicators change their smell when dipped in acid and base.
(a) Onion Extracts
- Retains its smell in acid and loses in base.
(b) Vanilla Extracts
- Retains its smell in acid and loses in base.
Table: Indicator Change in Acidic Medium
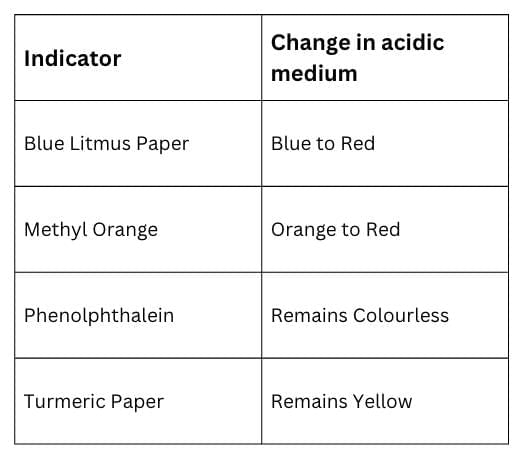
Table: Indicator Change in Basic Medium
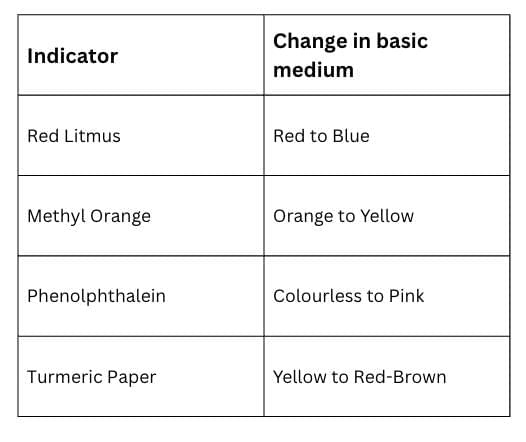
Some Solved Questions
Ques. What is an indicator?
Ans. An indicator is a dye that changes colour when it is put into an acid or a base. An indicator tells us whether a given substance is an acid or a base by change in its colour.
Ques. What is a universal indicator?
Ans. Universal indicator is a solution, which undergoes several colour, changes over a wide range of pH. The colour is used to ‘indicate’ pH directly. Universal indicators are usually mixtures of several indicators.
Ques. Name the common indicators used to test acid, bases and salts?
Ans. The three common indicators to test for acids and bases are
1) Litmus
2)Methyl Orange
3)Phenolphthalein
Ques.What are olfactory indicators. Give examples?
Ans. Those substances whose smell or odour changes in acidic or basic solution are called olfactory indicators.
Example: Onion and Vanilla extract
Ques. What is the colour of Phenolphthalein in acidic and basic medium?
Ans. It remains colourless in acids and turns pink in basic medium.
|
80 videos|569 docs|80 tests
|
FAQs on PH Scale: Indicators & Its Types - Science Class 10
| 1. What is the pH scale and how is it related to indicators? |  |
| 2. What are the different types of indicators? |  |
| 3. How does an indicator change in an acidic medium? |  |
| 4. How does an indicator change in a basic medium? |  |
| 5. How are indicators useful in determining the pH of a solution? |  |
















