Factors Affecting Equilibria & Le Chatelier's Principle | Chemistry Class 11 - NEET PDF Download
| Table of contents |

|
| Le Chatelier's Principle |

|
| Industrial Applications of Le Chatelier's Principle |

|
| Simultaneous Equilibrium |

|
| Heterogeneous reactions: the vapor pressure of solid hydrates |

|
Le Chatelier's Principle
If a system at equilibrium is subjected to a change of pressure, temperature, or the number of moles of a substance, there will be a tendency for a net reaction in the direction that tends to reduce the effect of this change.
Effect of Change in Concentration
The change in concentration can affect gaseous systems or liquid solution systems only. However, this does not affect the solid and pure liquid systems since their active masses are always taken as unity. By using Le Chatelier's principle, the effect of change in concentration on systems at equilibrium can be explained as follows:
1. When the concentration of reactant(s) is increased, the system tries to reduce their concentration by favoring the forward reaction.
2. When the concentration of product(s) is increased, the system tries to reduce their concentration by favoring the backward reaction.
3. When the concentration of reactant(s) is decreased, the system tries to increase their concentration by favoring the backward reaction.
4. When the concentration of product(s) is decreased, the system tries to increase their concentration by favoring the forward reaction.
When the concentration of reactants is increased, the number of effective collisions between them increases which in turn increases the rate of forward reaction. Thus the forward reaction is more favored over the backward reaction until the new equilibrium is established. At this new equilibrium, the rates of both forward and backward reactions become equal again and the reaction quotient becomes approximately equal to the equilibrium constant.
Remember that small changes in concentration do not affect the equilibrium constant.
For decomposition reaction , PCl5(g) PCl3(g) + Cl2(g)
Let the concentration of PCl5 is doubled to disturb the equilibrium. This will change the reaction quotient, Q to:
After disturbing the equilibrium, the value of Q becomes less than KC. To restore the Q value to KC, the concentration of PCl5 must be decreased while the concentrations of PCl3 and Cl2 are to be increased. This is achieved by favoring the forward reaction
The forward reaction is also favored by removing the products from the reaction mixture (decrease in the concentration of products). Upon removal of products, the rate of forward reaction becomes greater than that of backward reaction momentarily. This will also decrease the reaction quotient. Hence the system tries to reestablish the equilibrium by converting more reactants to products so as to make the rates of both forward and backward reactions become equal again.
For example, in the case of the decomposition of PCl5, if the concentration of Cl2 is increased by two times at equilibrium, the Q value becomes greater than the KC value.
Hence the system tries to restore the value of Q to KC again. The backward reaction is favored to decrease the concentration of Cl2. However, the concentration of PCl5 also decreases automatically while the concentration of PCl5 increases while doing so.
Effect of Change in Pressure
The change in pressure only affects the equilibrium of systems involving at least one gas. The Le Chatelier's principle can be applied to understand the effect of change in pressure on the systems at equilibrium as follows.
1. When the partial pressure of any of the gaseous reactants or of the products is increased, the position of equilibrium is shifted to decrease its partial pressure. This is usually achieved by favoring the reaction in which there is a decrease in the number of moles of gaseous components.
2. When the partial pressure of any of the gaseous reactants or of the products is decreased, the position of equilibrium is shifted so as to increase its partial pressure. This can be achieved by favoring that reaction in which there is an increase in the number of moles of gaseous components.
However, it is not always correct to say that the equilibrium is shifted whenever there is a change in the total pressure of the system. The equilibrium is not always disturbed upon changing the pressure of the entire system. It is only disturbed whenever there is a change in the partial pressure of any or all of the gaseous reactants or products in the equilibrium for which the Δng= 0. Where Δng = (no. of moles of gaseous products) - (no.of moles of gaseous reactants) Strictly speaking, the equilibrium is only shifted when the ratio of the product of partial pressures of products to the product of partial pressures of reactants i.e., the reaction quotient in terms of partial pressures, Qp is disturbed. The position of equilibrium is shifted to make Qp equal to the value of Kp again.
The Qp can be changed in the following cases:
1. By adding or removing any gaseous reactant or product at constant volume. The effect is the same as changing the concentration as explained above.
2. By changing the volume of the system (or in other words by changing the pressure of the entire system) at equilibrium for which the Δng = 0. In this case, however, the pressure of the entire system is also changed.
For the decomposition of PCl5, the Kp can be written as:
For this reaction Δng = (1 + 1)-(1) = 1
Hence for this reaction, if the pressure of the system is increased by 2 times by halving the volume, the reaction quotient, Qp is doubled. To restore back the Qp value again to Kp, the denominator value i.e., the partial pressure of PCl5 must be increased. This is only achieved by favoring the forward reaction in which less number of gaseous products are formed. i.e., Two moles of products (PCl3 and Cl2) are converted to one mole of reactant (PCl5)
Therefore we can say, that if the pressure of the system is increased, the system tries to decrease it by favoring the reaction in the direction of decreasing the number of moles of gaseous components.
Addition of Inert Gas
(A) At constant volume: there is no effect of adding inert gas on the state of equilibrium at constant volume.
(B) At constant pressure: On adding inert gas At constant pressure, the reaction proceeds in that direction where the sum of the stoichiometric coefficient of gaseous components is greater.
Effect of Change in Temperature
The effect of temperature can be understood by using Le Chatelier's principle as follows:
1. An increase in the temperature of the system favors the endothermic reaction. The increase in temperature increases the amount of heat in the system. Hence it tries to remove the excess of heat by favoring that reaction in which heat is absorbed i.e., the endothermic reaction.
2. A decrease in the temperature of the system favors the exothermic reaction. In this case, the temperature is decreased by removing the heat content from the system. Hence the system tries to restore the temperature back by favoring the exothermic reaction i.e., the reaction in which the heat is liberated.
It is very important to note that, during the temperature change, the system establishes a new equilibrium for which the value of the equilibrium constant is different from the original constant i.e., the equilibrium constant depends on the temperature.
Effect of Catalyst
A catalyst does not affect the position of the equilibrium since it increases not only the rate of forward reaction but also the rate of backward reaction. However, it does help the system to reach the equilibrium faster.
Industrial Applications of Le Chatelier's Principle
Haber Process
In the Haber process, the ammonia is synthesized by combining pure nitrogen and hydrogen gases in 1:3 ratio in the presence of a finely powdered iron catalyst and molybdenum promoter at around 450oC and at about 250 atm. of pressure.
The le Chatelier's principle helps in choosing these conditions to improve the yields of ammonia as explained below:
Effect of pressure
Hence the synthesis of ammonia is favored by increasing the pressure of the system. Industrially, 100 - 250 atm. of pressure is employed. Effect of temperature: Since the forward reaction is exothermic, the increase in temperature favors the backward reaction i.e., the dissociation of ammonia. That means according to Le Chatelier's principle, the synthesis of ammonia is favored at lower temperatures. However, the reaction will be too slow at lower temperatures (a kinetic restriction). Hence this reaction is carried out at optimal temperatures i.e., at about 450 - 550 oC to overcome the kinetic barrier. Removal of ammonia: The forward reaction can also be favored by removing ammonia from the system from time to time by liquefying it. Catalyst: To increase the speed of the reaction, finely powdered or porous iron is used as a catalyst. Its efficiency can be improved by adding molybdenum or oxides of potassium and aluminum.
The Percent by Mass of NH3 at Equilibrium in a Mixture of N2 ,H2 and NH3 as a Function of T and Total pressure | |||
|
| Total Pressure |
|
Temperature (°C) | 300 atm | 400 atm | 500 atm |
400 | 48% NH3 | 55% NH3 | 61% NH3 |
500 | 26% NH3 | 32% NH3 | 38% NH3 |
600 | 13% NH, | 17% NH, | 21% NH, |
Contact Process
In the contact process, sulfuric acid, the king of chemicals, is manufactured on a large scale. The major steps involved in the process are:
The crucial step is the oxidation of sulfur dioxide, SO2 to sulfur trioxide, SO3. It is a reversible reaction. At normal conditions, the equilibrium lies far to the left and the amount of sulfur trioxide formed is very small. To improve the yield of sulfur trioxide, the reaction is carried out at around 450oC and 2 atm pressure in the presence of V2O5 or Pt, which acts as catalysts.
These conditions are chosen by applying Le Chatelier's principle as explained below.
Effect of pressure: In the forward reaction (formation of sulfur trioxide), the number of moles of gaseous components decreases.
Hence the forward reaction is favored by increasing the pressure of the system. However, at high pressures, the iron towers used in the contact process are corroded. Hence the process is carried out at optimal pressures like 2 atm.
Effect of temperature: Since the forward reaction is exothermic, at higher temperatures the backward reaction i.e., the dissociation of sulfur dioxide is more favored. However, the reaction will be too slow at lower temperatures. Hence this reaction is carried out at optimal temperatures i.e., around 450° C.
Catalyst: To increase the speed of the reaction, V2O5 or Pt are used as catalysts
Ex. Under what conditions will the following reactions go in the forward direction?
1. N2(g) + 3H2(g) 2NH3(g) +23 Kcal.
2. 2SO2(g) + O2(g) 2SO3(g)+ 45 Kcal.
3. N2(g) + O2(g) 2NO(g) - 43.2 Kcal
4. 2NO(g) + O2(g) 2NO2(g)+ 27.8 Kcal
5. C(s) + H2O(g) CO2(g) + H2(g) + x k cal.
6. PCl5(g) PCl3(g) + Cl2(g) - X kcal.
7. N2O4(g) 2NO2(g) - 14 kcal
Sol. 1. Low T, High P, excess of N2 and H2 .
2. Low T, High P, excess of SO2 and O2
3. High T, any P , excess of N2 and O2
4. Low T, High P, excess of NO and O2
5. Low T, Low P, excess of C and H2O
6. High T, low P, excess of PCl5
7. High T, Low P, excess of N2O4
Simultaneous Equilibrium
A(s) B(g) + C(g)
D(s) B(g) + E(g)
Applicable only when at least one of the product is common in both the reaction.
Ex. The pressure at equilibrium over solid A is 50 atm and over solid D is 68 atm if both solid A and D are heated simultaneously then find
(i) the total pressure over the solids.
(ii) In the above question find the mole ratio of C and B
(iii) mole fraction of C
Sol. (i)
,
,
...(i)
...(ii)
total pressure = pB + pC + pE = x+ y+ x +y = 2 (x+ y)
Also
→
→ Total pressure = 2 (x + y) =
(ii) At constant temp and volume P ≈ n
→ Pressure ratio will their mole ratio by eq. (i)/(ii)
(iii) Mole fraction of C
As we know
→ →
,
,
→ mole fraction of C =
Ex.6 A(s) At eq., pressure = 18 atm
C(s) At eq., pressure = 36 atm
Calculation
(i) total pressure at new equilibrium when both the solids are heated simultaneously.
(ii) mole ratio of B and D
(iii) mole fraction of B in the mixture.
Sol. A(s) H2S(g) + B(g), Kp1 = (9)2 = 81
x + y x
C(s) H2S(g) + D(g), Kp2 = (18)2 = 324
x + y y
total pressure = x + y + x + y = 2 (x + y)
Kp1 = x(x + y)
Kp2 = y(x + y)
→ = (x + y)2 → x + y =
→ total pressure = 2 (x + y) = 2 =
(ii) mole ratio of B & D = =
=
(iii) mole fraction of B in mixture = =
Heterogeneous reactions: the vapor pressure of solid hydrates
Many common inorganic salts form solids which incorporate water molecules into their crystal structures.
These water molecules are usually held rather loosely and can escape as water vapor. Copper(II) sulfate, for example forms a pentahydrate in which four of the water molecules are coordinated to the Cu2 ion while the fifth is hydrogen-bonded to SO4--. This latter water is more tightly bound, so that the pentahydrate loses water in two stages on heating: CuSO4.5H2OCuSO4.H2O
CuSO4
These dehydration steps are carried out at the temperatures indicated above, but at any
temperature, some moisture can escape from a hydrate. For the complete dehydration of the
pentahydrate, we can define an equilibrium constant CuSO4.5H2O(s) → CuSO4(s) + 5 H2O(g) Kp = 1.14×10-10
The vapor pressure of the hydrate (for this reaction) is the partial pressure of water vapor at which the two solids can coexist indefinitely; its value is Kp1/5 atm. If a hydrate is exposed to air in which the partial pressure of water vapor is less than its vapor pressure, the reaction will proceed to the right and the hydrate will lose moisture. Vapor pressures always increase with temperature, so any of these compounds can be dehydrated by heating. Loss of water usually causes a breakdown in the structure of the crystal; this is commonly seen with sodium sulfate, whose vapor pressure is sufficiently large that it can exceed the partial pressure of water vapor in the air when the relative humidity is low. What one sees is that the well-formed crystals of the decahydrate undergo deterioration into a powdery form, a phenomenon known as efflorescence. When a solid is able to take up moisture from
the air, it is described as hygroscopic. A small number of anhydrous solids that have low vapor pressures not only take up atmospheric moisture on even the driest of days but will become wet as water molecules are adsorbed onto their surfaces; this is most commonly observed with sodium hydroxide and calcium chloride. With these solids, the concentrated solution that results continues to draw in water from the air so that the entire crystal eventually dissolves into a puddle of its own making; solids exhibiting this behavior are said to be deliquescent.
name | formula | vapor pressure, 25°C | Torr 30°C |
sodium sulfate decahydrate | Na2SO45H20 | 19.2 | 25.3 |
copper sulfate pentahydrate | CuS04-5H20 | 7.8 | 12.5 |
calcium chloride monohydrate | CaCl2H20 | 3.1 | 5.1 |
(water) | H20 | 23.5 | 31.6 |
One of the first hydrates to be investigated in detail was calcium sulfate hemihydrate (CaSO4×1/2 H2O) which LeChâtelier showed to be what forms when the form of CaSO4 known as plaster of Paris hardens; the elongated crystals of the hydrate bind themselves into a cement-like mass.
Ex. At what relative humidity will copper sulfate pentahydrate lose its waters of hydration when the air temperature is 30°C? What is Kp for this process at this temperature?
Sol. From the table, we see that the vapor pressure of the hydrate is 12.5 torr, which corresponds to a relative humidity of 12.5/31.6 = 0.40 or 40%. This is the humidity that will be maintained if the hydrate is placed in a closed container of dry air.
For this hydrate, Kp = 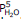 , so the partial pressure of water vapor that will be in equilibrium with the hydrate and the dehydrated solid (remember that both solids must be present to have equilibrium!), expressed in atmospheres, will be (12.5/760)5= 1.20 x10-9.
, so the partial pressure of water vapor that will be in equilibrium with the hydrate and the dehydrated solid (remember that both solids must be present to have equilibrium!), expressed in atmospheres, will be (12.5/760)5= 1.20 x10-9.
|
114 videos|263 docs|74 tests
|
FAQs on Factors Affecting Equilibria & Le Chatelier's Principle - Chemistry Class 11 - NEET
| 1. What is Le Chatelier's Principle and how is it applied in industrial processes? |  |
| 2. How is Le Chatelier's Principle used in the production of ammonia through the Haber process? |  |
| 3. What are some industrial applications of Le Chatelier's Principle other than the Haber process? |  |
| 4. How does Le Chatelier's Principle help in the optimization of industrial reactions? |  |
| 5. What are the benefits of applying Le Chatelier's Principle in industrial processes? |  |





















