Interpreting Mass Spectra | Chemistry Optional Notes for UPSC PDF Download
Interpreting Mass Spectra
What kinds of information can we get from a mass spectrum? The most obvious information is the molecular weight of the sample, which in itself can be invaluable. If we were given samples of hexane (MW = 86), 1-hexene (MW = 84), and 1-hexyne (MW = 82), for example, mass spectrometry would easily distinguish them.
Some instruments, called double-focusing mass spectrometers, have two magnetic sectors in their mass analyzers, giving these spectrometers have such high resolution that they provide mass measurements accurate to 5 ppm, or about 0.0005 amu, making it possible to distinguish between two formulas with the same nominal mass. For example, both C5H12 and C4H8O have MW = 72, but they differ slightly beyond the decimal point: C5H12 has an exact mass of 72.0939 amu, whereas C4H8O has an exact mass of 72.0575 amu. A high-resolution instrument can easily distinguish between them. Note, however, that exact mass measurements refer to molecules with specific isotopic compositions. Thus, the sum of the exact atomic masses of the specific isotopes in a molecule is measured—1.007 83 amu for 1H, 12.000 00 amu for 12C, 14.003 07 amu for 14N, 15.994 91 amu for 16O, and so on—rather than the sum of the average atomic masses of elements, as found on a periodic table.
Unfortunately, not every compound shows a molecular ion in its electron-impact mass spectrum. Although M+ is usually easy to identify if it’s abundant, some compounds, such as 2,2-dimethylpropane, fragment so easily that no molecular ion is observed (Figure 12.5). In such cases, alternative “soft” ionization methods that don’t use electron bombardment can prevent or minimize fragmentation (see Section 12.4).
Knowing the molecular weight makes it possible to narrow considerably the choices of molecular formula. For example, if the mass spectrum of an unknown compound shows a molecular ion at m/z = 110, the molecular formula is likely to be C8H14, C7H10O, C6H6O2, or C6H10N2. There are always a number of molecular formulas possible for all but the lowest molecular weights, and a computer can easily generate a list of the choices.
A further point about mass spectrometry, noticeable in the spectra of both propane (Figure 12.4) and 2,2-dimethylpropane (Figure 12.5), is that the peak for the molecular ion is not at the highest m/z value. There is also a small peak at M + 1 due to the presence of different isotopes in the molecules. Although 12C is the most abundant carbon isotope, a small amount (1.10% natural abundance) of 13C is also present. Thus, a certain percentage of the molecules analyzed in the mass spectrometer are likely to contain a 13C atom, giving rise to the observed M + 1 peak. In addition, a small amount of 2H (deuterium; 0.015% natural abundance) is present, making a further contribution to the M + 1 peak.
Mass spectrometry would be useful even if molecular weight and formula were the only information that could be obtained, but in fact it provides much more. For one thing, the mass spectrum of a compound serves as a kind of “molecular fingerprint.” Every organic compound fragments in a unique way depending on its structure, and the likelihood of two compounds having identical mass spectra is small. Thus, it’s sometimes possible to identify an unknown by computer-based matching of its mass spectrum to one of the more than 785,061 searchable spectra recorded in a database called the Registry of Mass Spectral Data.
It’s also possible to derive structural information about a molecule by interpreting its fragmentation pattern. Fragmentation occurs when the high-energy cation radical flies apart by spontaneous cleavage of a chemical bond. One of the two fragments retains the positive charge and is a carbocation, while the other fragment is a neutral radical.
Not surprisingly, the positive charge often remains with the fragment that is best able to stabilize it. In other words, a relatively stable carbocation is often formed during fragmentation. For example, 2,2-dimethylpropane tends to fragment in such a way that the positive charge remains with the tert-butyl group. 2,2-Dimethylpropane therefore has a base peak at m/z = 57, corresponding to C4H9+ (Figure 12.5).
Because mass-spectral fragmentation patterns are usually complex, it’s often difficult to assign structures to fragment ions. Most hydrocarbons fragment in many ways, as demonstrated by the mass spectrum of hexane in Figure 12.6. The hexane spectrum shows a moderately abundant molecular ion at m/z = 86 and fragment ions at m/z = 71, 57, 43, and 29. Since all the carbon–carbon bonds of hexane are electronically similar, all break to a similar extent, giving rise to the observed mixture of ions.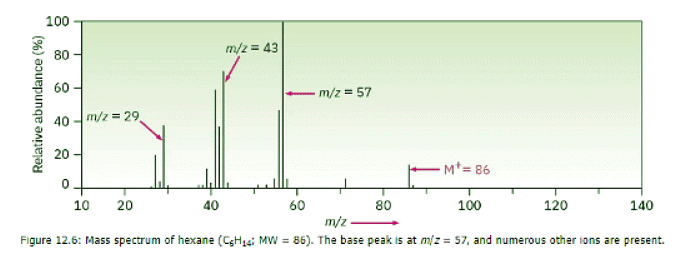 Figure 12.7 shows how the hexane fragments might arise. The loss of a methyl radical (CH3, M = 15) from the hexane cation radical (M+ = 86) gives rise to a fragment of mass 86 – 15 = 71; the loss of an ethyl radical (C2H5, M = 29) accounts for a fragment of mass 86 – 29 = 57; the loss of a propyl radical (C3H7, M = 43) accounts for a fragment of mass 86 – 43 = 43; and the loss of a butyl radical accounts for a fragment of mass 29. With practice, it’s sometimes possible to analyze the fragmentation pattern of an unknown compound and work backward to a structure that is compatible with the data.
Figure 12.7 shows how the hexane fragments might arise. The loss of a methyl radical (CH3, M = 15) from the hexane cation radical (M+ = 86) gives rise to a fragment of mass 86 – 15 = 71; the loss of an ethyl radical (C2H5, M = 29) accounts for a fragment of mass 86 – 29 = 57; the loss of a propyl radical (C3H7, M = 43) accounts for a fragment of mass 86 – 43 = 43; and the loss of a butyl radical accounts for a fragment of mass 29. With practice, it’s sometimes possible to analyze the fragmentation pattern of an unknown compound and work backward to a structure that is compatible with the data.
Solved Example
Example: Using Mass Spectra to Identify Compounds
Assume that you have two unlabeled samples, one of methylcyclohexane and the other of ethylcyclopentane. How could you use mass spectrometry to tell them apart? The mass spectra of both are shown in Figure 12.8.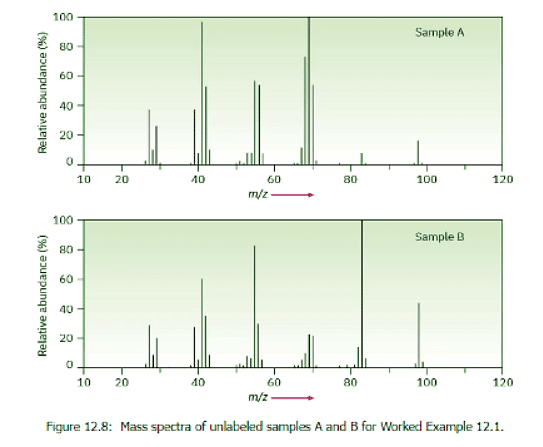 Ans: Strategy: Look at the possible structures and decide on how they differ. Then think about how any of these differences in structure might give rise to differences in mass spectra. Methyl cyclohexane, for instance, has a –CH3 group, and ethylcyclopentane has a –CH2CH3 group, which should affect the fragmentation patterns.
Ans: Strategy: Look at the possible structures and decide on how they differ. Then think about how any of these differences in structure might give rise to differences in mass spectra. Methyl cyclohexane, for instance, has a –CH3 group, and ethylcyclopentane has a –CH2CH3 group, which should affect the fragmentation patterns.
Solution: Both mass spectra show molecular ions at M+ = 98, corresponding to C7H14, but they differ in their fragmentation patterns. Sample A has its base peak at m/z = 69, corresponding to the loss of a CH2CH3 group (29 mass units), but B has a rather small peak at m/z = 69. Sample B shows a base peak at m/z = 83, corresponding to the loss of a CH3 group (15 mass units), but sample A has only a small peak at m/z = 83. We can therefore be reasonably certain that A is ethylcyclopentane and B is methylcyclohexane.
FAQs on Interpreting Mass Spectra - Chemistry Optional Notes for UPSC
| 1. What is mass spectrometry and how is it used in interpreting mass spectra? |  |
| 2. What are the main components of a mass spectrometer? |  |
| 3. How can mass spectra be used to determine the molecular weight of a compound? |  |
| 4. What is fragmentation in mass spectrometry and how does it help in identifying compounds? |  |
| 5. How can isotopic patterns be used in interpreting mass spectra? |  |

|
Explore Courses for UPSC exam
|

|
















