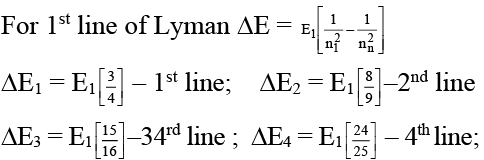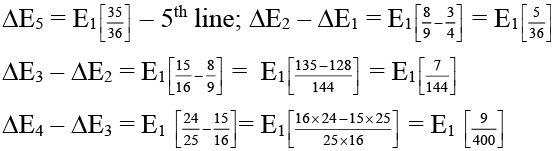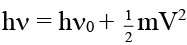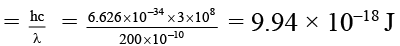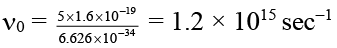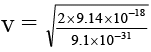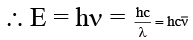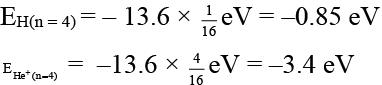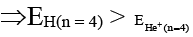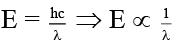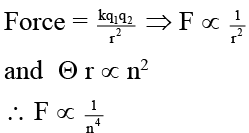JEE Advanced (One or More Correct Option): Structure of Atom | Chapter-wise Tests for JEE Main & Advanced PDF Download
Q.1. Which of the following statements are correct?
(a) The total spectral lines obtained from a single line during Zeeman effect is (2l + 1).
(b) In the Lyman series as the energy liberated during transition increases then the distance between the spectral lines goes on decreasing.
(c) The highest probability of finding an electron in 1s orbital is in the vicinity of the circumference.
(d) The highest probability of finding an electron in 1s orbital is exactly at the middle between nucleus and circumference.
Correct Answer is option (a, b and c)
(A) The total lines obtained due to the splitting of a spectral line in the presence of magnetic effect is (2l + 1) as the presence of orbitals which have specific orientation in the presence of external field take up certain new orientation. The number of orbitals are equal to (2l + 1) and for each orbital one splitting takes place.
(B) As we move away from the nucleus the difference in the energy levels become lesser and lesser hence.
We find that (ΔE2 - ΔE1) > (ΔE3 - ΔE2) > (ΔE4 - ΔE3) Hence the distance also become lesser and lesser.
(C) The distance from the nucleus for maximum probability of finding electrons is 0.53 Å. This is not on the circumference of the orbital but is in the vicinity of the circumference.
Q.2. Radiation of wavelength 200 Å falls on a platinum surface. If the work function of the metal is 5 eV. Which of the following results are correct about experiment?
(a) The velocity of photoelectrons increases with increase in intensity of radiation
(b) Photo-emission of electrons takes place
(c) the threshold frequency of the metal is 1.21 × 1015 sec–1
(d) the velocity of the photo-electrons is 4.48 × 106 m/sec.
Correct Answer is option (b, c and d)
According to emission photoelectric effect
E of incident radiation
1/2 mv2 = 9.94 × 10–18 – 8 × 10–19 = 9.14 × 10–18
= 4.47 × 106 m/sec
Q.3. Energy equivalent of 10.00 cm–1 is –
(a) 2 × 10–22 J per photon
(b) 2.9 × 10–2 kcal mol–1 photon
(c) 1.2 × 10–1 kJ mol–1 photon
(d) 2 × 10–15 ergs per photon
Correct Answer is option (a, b, c and d)
10 cm–1 = 1000 m–1
= 6.66 × 10–34 (Js) × 3 × 108 (ms–1) × 1000 (m–1)
= 2 × 10–22 J per photon = 2 × 10–15 ergs per photon
= 2 × 10–22 × 6.02 × 1023 J mol–1
= 1.2 × 10–1 kJ mol–1
= 2.9 × 10–2 kcal mol–1
Q.4. Identify the correct statement (s):
(a) Energy of the electron in 4th orbit of H-atom is more than that of the electron present in 4th orbit of He+ion
(b) Energy of a quantum is inversely proportional to frequency (ν)
(c) Energy of a quantum is inversely proportional to wavelength (λ)
(d) Radius of Ist orbit of He+ ion is 0.529 Å
Correct Answer is option (a and c)
(a)
So – 0.85 > – 3.4
(c)
Q.5. Which of the following statement (s) is/are correct?
(a) Fe3+ and Mn2+ have equal paramagnetic character
(b) Cu2Cl2 and CuCl2 are coloured.
(c) MnO4- is purple in colour because of unpair d electrons
(d) The magnetic moment of Fe2+ and CO3+ both are equal to 2√6 B. M.
Correct Answer is option (a and d)
Both Fe3+ & Mn2+ have 5 unpair electrons
Cu2Cl2 is colourless because Cu+ has no unpair electrons
MnO4- has no unpair electrons in Mn+7, due to charge transfer spectra it is coloured.
Q.6. In which of the following elements Aufbau principle is/are not followed?
(a) Tc (43)
(b) Ru (44)
(c) Rh (45)
(d) Pd (46)
Correct Answer is option (b, c and d)
In Tc : 4 d5 5s2 Aufbau principle is followed
In Ru : 4 d7 5s1 Aufbau principle is not followed
In Rh : 4 d8 5s1 Aufbau principle is not followed
In Pd : 4 d10 Aufbau principle is not followed
Q.7. Choose the correct relations on the basis of Bohr's theory
(a) Velocity of an electron ∝ 1/n
(b) Frequency of resolutions ∝ 1/n3
(c) Radius of orbit ∝ n2Z
(d) Force on an electron ∝ 1/n4
Correct Answer is option (a, b and d)
Q.8. Which of the following species have magnetic moment 4.89 B.M.
(a) Fe+2
(b) Co+3
(c) Ni+4
(d) Mn+2
Correct Answer is option (a, b and c)
Magnetic moment = 4.89 BM, so the species must have 4 unpaired e–s.
In Fe+2, Co3+, Ni+4 number of unpaired e – s = 4 in their 3d - subshell.
Where as in Mn+2 there are 5 unpaired e–s in 3d subshell.
Q.9. An electron is excited from the ground state to the n = 3 state in hydrogen atom. Which of the following statements are true?
(a) It takes more energy to ionize (remove) the electron from n = 3 then from the ground state
(b) The electron is farther from the nucleus on average in the n = 3 state then in the ground state.
(c) The wavelength of light emitted if the electron drops from n = 3 to n = 2 is longer than the wavelength of the light emitted it the electron falls from n = 3 to n = 1.
(d) The first excited state corresponds to n = 3.
Correct Answer is option (b and c)
(a) It is easier to ionize electron from 3rd orbit than ground state
(b) The radius of the third orbit is more than first orbit.
(c) The wave length of Balmer series (n2 → 2) is more than lyman series (n2 → 1).
(d) The first excited state compounds to n = 2.
Q.10. 0.53 Å is Bohr’s radius of the first orbit. In the light of the wave mechanical model, it suggests that
(a) The product of ψ2 and 4πr2 dr increases till it reaches at the distance of 0.53 Å.
(b) Only ψ2 goes on increasing, 4πr2 dr remains constant till it reaches at the distance of 0.53 Å.
(c) ψ2 goes on increasing, 4πr2 dr goes on decreasing till it reaches at the distance of 0.53 Å.
(d) only 4πr2 dr goes on increasing, ψ2 remains constant till it reaches at the distance of 0.53 Å.
Correct Answer is option (a)
The probability of finding 1s electron, is maximum near nucleus. On moving farther to nucleus close to 0.53 Å ( Bohr’s radius) ψ2 decreases. 4π2 dr, the volume element, on moving farter to nucleus increases till it reaches at Bohr's radius and then starts to decrease.
|
446 docs|930 tests
|