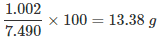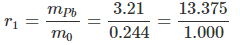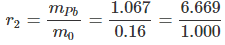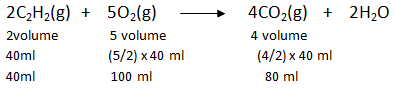Laws of Chemical Combinations | Chemistry Class 11 - NEET PDF Download
What are the Laws of Chemical Combinations?
The combination of different elements to form compounds is governed by certain basic rules. These rules are referred to as laws of chemical combination.
- In chemistry, we study the change of matter from one form to the other. These changes sometimes occur as a result of the combination of two different types of matter.
- There are certain rules which are followed in the combination of different elements to form different compounds.
There are five laws of chemical combinations:
- Law of Conservation of Mass
- Law of Definite Proportions
- Law of Multiple Proportions
- Gay Lussac's Law of Gaseous Volumes
- Avogadro's Law
1. Law of Conservation of Mass
“The mass in an isolated system can neither be created nor be destroyed but can be transformed from one form to another”. Let us look at an example first-
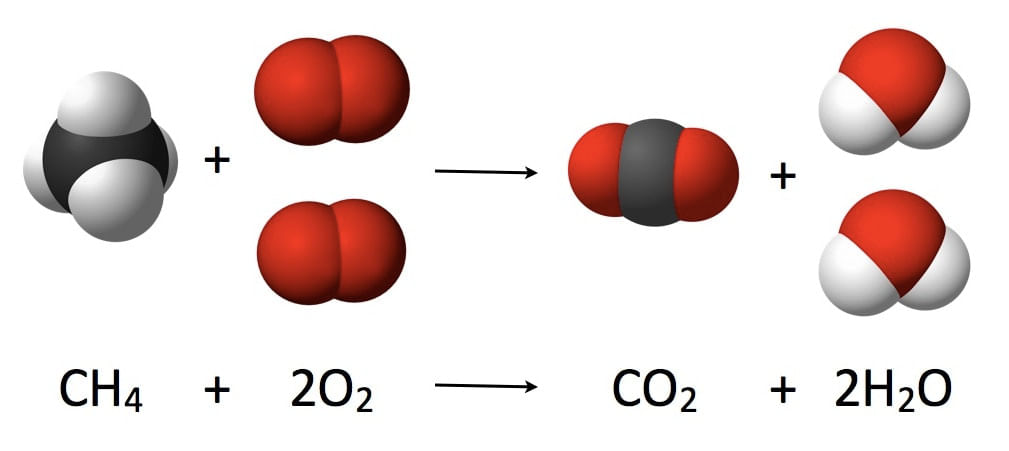 No. of Carbons, Hydrogens and Oxygens are the same on each side of the equation.
No. of Carbons, Hydrogens and Oxygens are the same on each side of the equation.
- In the above experiment, Mass of Reactants = Mass of Products which follows Law of Conservation of Mass.
Limitation of Law of Conservation of Mass
- In nuclear reactions, some mass of reactant is converted into energy, so the mass of reactant is always less than that of product.
- The other limitation of conservation of mass is according to Einstein’s theory, the relation between two quantities is given by E = mc2, which means that energy and mass are interconvertible. Therefore, for the law of conservation of mass to be valid mass and energy of the system must be conserved.
Solved Examples of Law of Conservation of Mass
Example 1: A 15.9 gm sample of sodium carbonate is added to a solution of acetic acid weighing 20.0 gm. The two substances react, releasing carbon dioxide gas to the atmosphere. After the reaction, the contents of the reaction vessel weigh 29.3 gm. What is the mass of carbon dioxide given off during the reaction?
The total mass of reactants taken = 15.9 + 20.0 = 35.9 gm.
- From the conservation of mass, the final mass of the contents of the vessel should also be 35.9 gm.
- But it is only 29.3 gm. The difference is due to the mass of released carbon dioxide gas.
- Hence, the mass of carbon dioxide gas released = 35.9 - 29.3 = 6.6 gm.
Example 2: 5.2 g of CaCO3 when heated produced 1.99 g of Carbon dioxide and the residue (CaO) left behind weighs 3.2 g. Show that these results illustrate the law of conservation of mass.
Weight of CaCO3 taken = 5.2 g
- Total weight of the products (CaO + CO2 )= 3.20 + 1.99 = 5.19 g
- Difference between the wt. of the reactant and the total wt. of the products
= 5.20 – 5.19 =0.01 g.- This small difference may be due to experimental error.
- Thus the law of conservation of mass holds good within experimental errors.
2. Law of Definite Proportions
"All chemical compounds are found to have constant composition irrespective of their method of preparation or sources."
- In the year 1794, the French chemist Joseph Proust formulated the law of constant proportions from his work on sulphides, metallic oxides, and sulfates.
- In H2O, Hydrogen & oxygen combine in a 2:1 molar ratio, this ratio remains constant whether it is Tap water, river water, or seawater or produced by any chemical reaction.
 The elements are always combined in the same proportions by mass.
The elements are always combined in the same proportions by mass.
Limitations of Law of Definite Proportions
- The law does not hold true if the different isotopes of the same element are involved in making chemical compounds.
- In the case of Isotopes, Combining Ratio is not fixed
Example:
12CO2 14CO2
12:32 14:323:8 7:16
Tip: Isotopes are the atoms possessing the same atomic number but different atomic masses.
Solved Examples of Law of Definite Proportions
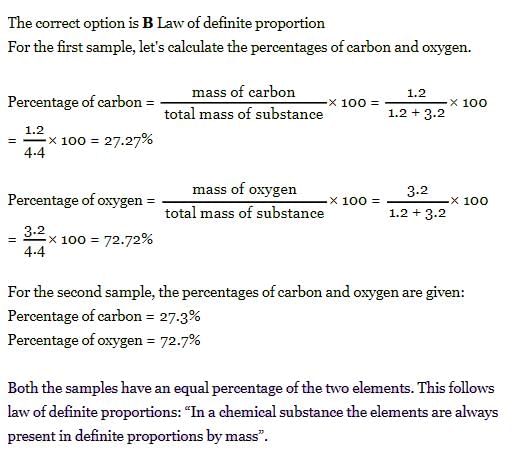
Example 1: The following are results of the analysis of two samples of the same or two different compounds of phosphorus and chlorine. From these results, decide whether the two samples from the same or different compounds. Also state the law, which will be obeyed by the given samples.
- The mass ratio of phosphorus and chlorine in compound A:
mp : mcl = 1.156 : 3.971 = 0.2911 : 1.000- The mass ratio of phosphorus and chlorine in compound B:
mp : mcl = 1.542 : 5.297 = 0.2911 : 1.000
As the mass ratio is the same, both the compounds are the same and the samples obey the law of definite proportion.
Example 2: 6.488 g of lead combine directly with 1.002 g of oxygen to form lead peroxide PbO2. Lead peroxide is also produced by heating lead nitrate and it was found that the percentage of oxygen present in lead peroxide is 13.38 percent. Use these data to illustrate the law of constant composition.
Step 1:
- To calculate the percentage of oxygen in the first experiment.
- Weight of peroxide formed = 6.488 + 1. 002 = 7.490 g
- 7. 490 g of lead peroxide contain 1.002 g of oxygen
∴ 100 g of lead peroxide will contain oxygen:
i.e. oxygen present = 13.38%Step 2:
- To compare the percentage of oxygen in both experiments. Percentage of oxygen in PbO2 in the first experiment = 13.38
- Since the percentage composition of oxygen in both the samples of PbO2 is identical, the above data illustrate the constant composition law.
3. Law of Multiple Proportions
"When one element combines with the other elements to form two or more different compounds, the mass of one element, which combines with a constant mass of the other, bears a simple ratio to one another."
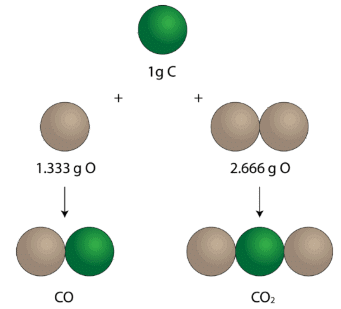 Carbon can form two different compounds with oxygen
Carbon can form two different compounds with oxygen
- Carbon is found to form two oxides containing 42.9% & 27.3% of carbon, respectively, showing that these figures show the law of multiple proportions.

In the first oxide:
- 57.1 parts by mass of oxygen combine with 42.9 parts of carbon.
- 1 part of oxygen will combine with 42.9/57.1 = 0.751 part of the carbon.
Similarly, in 2nd oxide:
- 1 part of oxygen will combine with 27.3/72.7 = 0.376 part of the carbon.
- The ratio of carbon that combines with the same mass of oxygen = 0.751: 0.376 = 2:1
- This is a simple whole-number ratio, this means the above data shows the law of multiple proportions.
Limitations of Law of Multiple Proportions
- The law of multiple proportions is not widely true or acceptable.
- The law is only applicable to small Reactions.
It means that this law is valid for basic chemical reactions.
The Law of multiple proportions is not valid for heavy and complex molecules
Because when heavy molecules are interlinked with each other then the total whole will not be as small.
Solved Examples of Law of Multiple Proportions
Example 1: Two oxide samples of lead were heated in the current of hydrogen and were reduced to metallic lead. The following data were obtained:
(i) Weight of yellow oxide taken = 3.45 gm; Loss in weight during reduction = 0.24 gm
(ii) Weight of brown oxide taken = 1.227 gm; Loss in weight during reduction = 0.16 gm.
Show that the data illustrate the law of multiple proportions.
- When the oxide of lead is reduced in the current of hydrogen, metallic lead is formed.
- Definitely, the loss in weight is due to the removal of the oxygen present in the oxide, to combine with the hydrogen.
- Therefore, the composition of the yellow oxide is:
Oxygen = 0.24 gm and lead = 3.45 - 0.24 = 3.21 gm.- The mass ratio of lead and oxygen:
- The composition of the brown oxide is : oxygen = 0.16 gm and lead = 1.227 - 0.16 = 1.067 gm.
- The mass ratio of lead and oxygen:
- Now, r1 : r2 = 13.375 : 6.669 = 2:1 (simple ratio) and hence the data illustrates the law of multiple proportion.
Example 2: Carbon is found to form two oxides, which contain 42.9% and 27.3% of carbon respectively. Show that these figures illustrate the law of multiple proportions.
Step 1:
- To calculate the percentage composition of carbon and oxygen in each of the two oxides:
- First oxide: Carbon 42.9% 27.3% (Given)
- Second oxide: Carbon Oxygen 57.1% 72.7% (by difference)
Step 2:
To calculate the weights of carbon which combine with a fixed weight i.e., one part by weight of oxygen in each of the two oxides.
- In the first oxide, 57.1 parts by weight of oxygen combine with carbon = 42.9 parts.
∴ 1 part by weight of oxygen will combine with carbon 42.9/57.1 = 0.751- In the second oxide, 72.7 parts by weight of oxygen combine with carbon = 27.3 parts.
∴ 1 part by weight of oxygen will combine with carbon 27.3/72.7 = 0.376Step 3:
- To compare the weights of carbon, combined with the same weight of oxygen in both the oxides.
- The ratio of the weights of carbon that combine with the same weight of oxygen (1 part) is 0.751: 0.376 or 2:1
Since this is a simple whole-number ratio, so the above data illustrate the law of multiple proportions.
4. Gay Lussac's Law of Gaseous Volumes
- The law was proposed by Gay Lussac in 1808.
- When gases combine or are produced in a chemical reaction, they do so in a simple ratio by volume.
- This ratio holds true when all gases involved are at the same temperature and pressure.
- For instance, when hydrogen and oxygen react to form water vapor, 100 mL of hydrogen combines with 50 mL of oxygen to produce 100 mL of water vapor.
- The volumes of hydrogen and oxygen in this example (100 mL and 50 mL) bear a simple ratio of 2:1.
- Gay Lussac's discovery is an extension of the law of definite proportions, which was initially stated for mass.
- Avogadro further explained Gay Lussac's law in 1811, emphasizing the relationship between volume and the number of particles.
- Gay Lussac's law describes how gases combine in simple volume ratios under specific conditions, contributing to our understanding of chemical reactions. Avogadro's work later provided a more comprehensive explanation of this law.
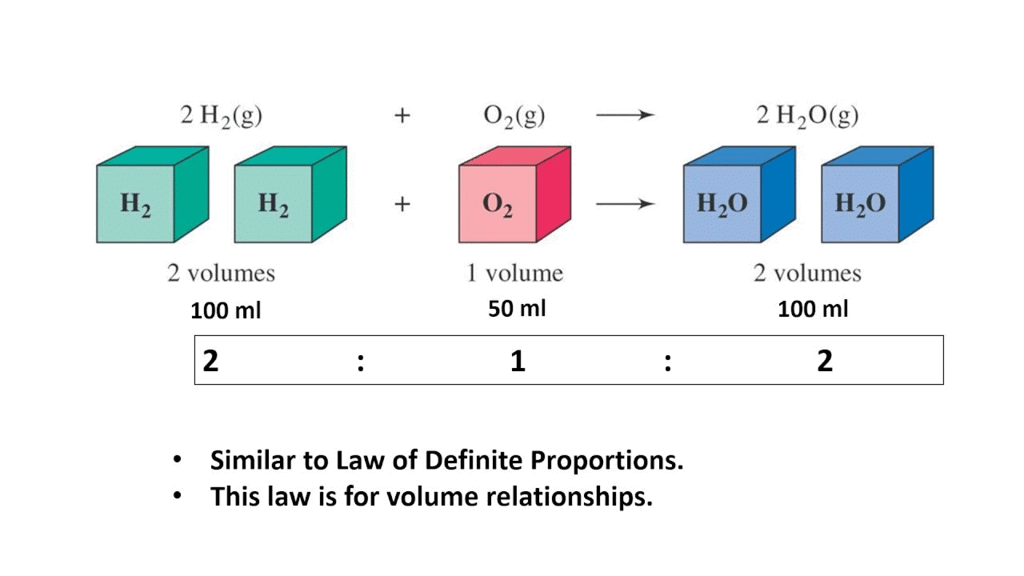 Example of Gay Lussac's Law
Example of Gay Lussac's Law
Limitations of Gay-Lussac's Law
- The law is only applicable to ideal gases.
- Gay-Lussac's law holds good for real gases at high temperatures and/or low pressure.
- The ratio of the pressure to temperature deviated at high pressures. The ratio decreases with increasing the pressure.
Solved Examples of Gay-Lussac's Law
Example 1: 2.5 ml of a gaseous hydrocarbon exactly requires 12.5 ml oxygen for complete combustion and produces 7.5 ml carbon dioxide and 10.0ml water vapour. All the volumes are measured at the same pressure and temperature. Show that the data illustrates Gay Lussac's law of volume combination.
V(hydrocarbon) : Voxygen : V(carbon dioxide) : V(water vapour)
= 2.5 : 12.5 : 7.5 : 10.0
= 1 : 5 : 3 : 4 (simple ratio)Hence, the data is according to the law of volume combination.
Example 2: How much volume of oxygen will be required for the complete combustion of 40 ml of acetylene (C2H2) and how much volume of carbon dioxide will be formed? All volumes are measured at NTP.
So, for complete combustion of 40 ml of acetylene, 100 ml of oxygen is required and 80 ml of CO2 is formed.
5. Avogadro's Law
"Equal volumes of gases at the same temperature and pressure should contain an equal number of molecules."
- This implies that 2 litres of hydrogen will have the same number of molecules as 2 litres of oxygen given that both gases are at the same temperature and pressure.
- Avogadro made a distinction between atoms and molecules which is quite understandable in the present times.
- If we consider the reaction of hydrogen and oxygen to produce water, we see that two volumes of hydrogen combine with one volume of oxygen to give two volumes of water without leaving any unreacted oxygen.
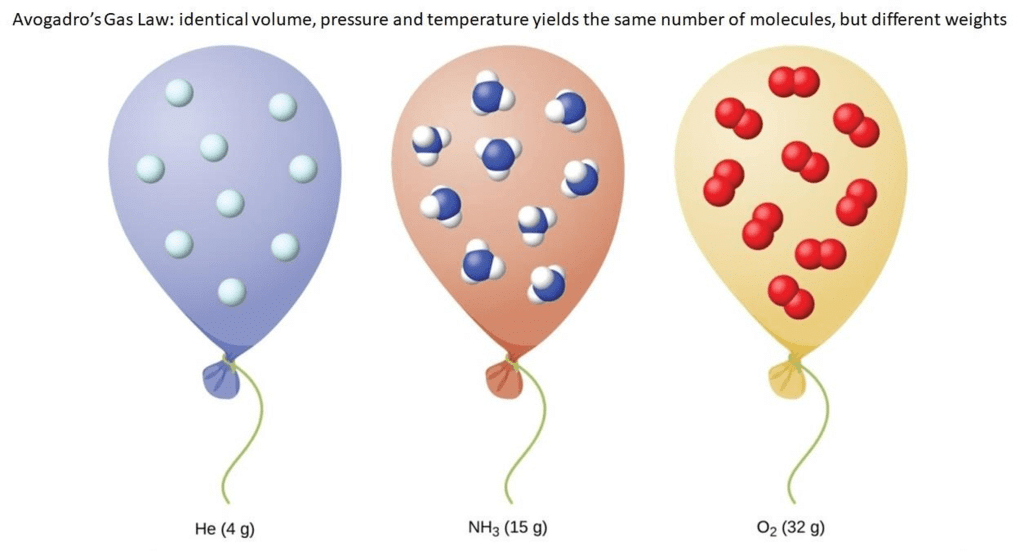 Avogadro's Law
Avogadro's Law
Limitations of Avogadro's Law
- The law works perfectly only for ideal gases.
- The law is approximate for real gases at low pressure and/or high temperature.
Solved Example of Avogadro's Law
Examples 1: A tyre that consists of 10 moles air and occupies 40L volume loses half of the volume because of a puncture. What is the amount of air left in the deflated tyre at STP?
Initial amount of air n1 is 10 mol
Initial volume of tyre V1 is 40 L
Final volume of tyre V2 is 20 L
According to Avogadro’s law,
the amount of air left in the deflated tyre,
n2 =
= 5 moles
Hence, the deflated tyre contains 5 moles air
Laws of Chemical Combinations: Solved Examples
Q1. What mass of silver nitrate will react with 5.85 g of sodium chloride to produce 14.35 g of silver chloride and 8.5 g of sodium nitrate, if the law of conservation of mass is true?
According to the law of conservation of mass, the mass of the products in a chemical reaction must equal the mass of the reactants.
For the chemical reaction: AgNO3 + NaCI → NaNO3 + AgCI
mreactants = mproducts
mAgNO3 + mNaCl = mNaNO3 + mAgCl
x + 5.85 = 8.5 + 14.35
x = 17.0 g
Q.2. Elements X and Y form two different compounds. In the first, 0.324 g of X is combined with 0.471 g of Y. In the second, 0.117 g of X is combined with 0.509 g of Y. Show that these data illustrate the law of multiple proportions.
Ans: According to the law of multiple proportions, "If two elements form more than one compound between them, then the ratios of the masses of the second element which combine with a fixed mass of the first element will be ratios of small whole numbers".
- In the first compound, 0.324 g of X is combined with 0.471 g of Y.
Hence, 1 g of X is combined with 0.471/0.324 = 1.45 g of Y.- In the second compound, 0.117 g of X is combined with 0.509 g of Y.
Hence, 1 g of X is combined with 0.1170.509 = 4.35 g of Y.- The ratio of 4.35/1.35 = 3 is a small whole number.
|
121 videos|348 docs|74 tests
|
FAQs on Laws of Chemical Combinations - Chemistry Class 11 - NEET
| 1. What is the Law of Conservation of Mass in chemical combinations? |  |
| 2. How does the Law of Definite Proportions apply in chemical combinations? |  |
| 3. What is Gay Lussac's Law of Gaseous Volumes in chemical combinations? |  |
| 4. How does Avogadro's Law relate to chemical combinations? |  |
| 5. Can you explain the Law of Multiple Proportions in chemical combinations? |  |