Class 7 Science Chapter 4 Question Answers - Acids, Base and Salts
Q1. Write the characteristics of acids by which we can identify acids.
Ans: Acids are unique substances that possess particular characteristics which make them identifiable.
- Firstly, one of the most notable properties of acids is their sour taste. This characteristic is what gives citrus fruits their tangy flavor as they contain citric acid.
- Secondly, another significant characteristic of acids is their ability to turn blue litmus paper or solution to red. Litmus paper is a pH indicator used to test whether a solution is acidic or alkaline. When an acid is introduced to blue litmus paper, it changes its color to red, indicating the presence of an acid.
- Lastly, the dilution of acid in water results in an exothermic reaction. This means that the process produces or releases heat energy. Therefore, these characteristics play a crucial role in the identification of acids.
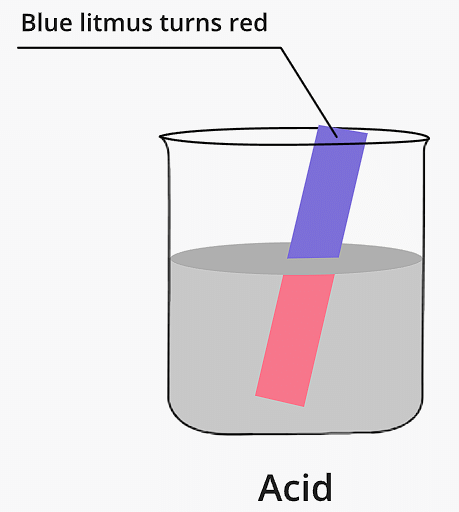 Blue Litmus paper turns red
Blue Litmus paper turns red
Q2. A small amount of hydrochloric acid is always produced in the stomach. Is it useful or harmful for us? If excess of acid is produced in the stomach, what should we do?
Ans: The human body is a complex system that requires a balance of various substances to function correctly. One such substance is hydrochloric acid, which is produced in small amounts in the stomach.
- This acid is beneficial as it helps to kill off any potentially harmful bacteria that might have entered the stomach with the food we consume, thereby protecting us from bacterial infections.
- However, if the production of this acid exceeds the normal levels, it can prove to be harmful. Excess hydrochloric acid in the stomach can lead to a burning sensation or discomfort known as acid reflux or heartburn.
- In such situations, one can take an antacid medicine like milk of magnesia. Antacids work by neutralizing the excess acid in the stomach, thus providing relief from the discomfort.
Q3. Why we take an antacid tablet when we suffer from acidity?
Ans: Our stomach contains hydrochloric acid. It helps us to digest food. But too much of acid in the stomach causes indigestion. Sometimes indigestion is painful. To relieve indigestion, we take an antacid such as milk of magnesia, which contains magnesium hydroxide. It neutralises the effect of excessive acid.
Q4. After carrying out the neutralisation reaction, the test tube immediately found to be somewhat hot. Explain why.
Ans: The phenomenon observed during a neutralization reaction where the test tube becomes hot is due to the release of heat energy.
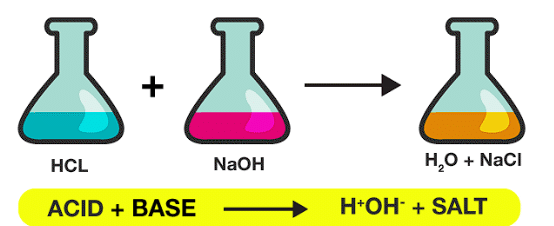
- A neutralization reaction is a type of chemical reaction that occurs between an acid and a base, resulting in the formation of a salt and water.
- This reaction is always exothermic, meaning it produces or releases heat. The heat evolved from the reaction elevates the temperature of the reaction mixture.
- Hence, if you touch the test tube immediately after the neutralization reaction, you will find it to be hot due to the heat energy evolved during the reaction.
Acid + Base → Salt + Water (heat is evolved)
Q5. How lime water is prepared in the laboratory?
Ans: Lime water, also known as calcium hydroxide solution, is prepared in the laboratory using a simple method.
- Firstly, add some lime or chuna to water in a bottle. Stir the solution thoroughly and let it sit for a while. As it settles, the lime particles dissolve in the water, creating
- To ensure that the solution is free from undissolved particles, pour out a little more from the top. This freshly prepared solution is lime water. It is often used in various scientific experiments due to its ability to react with acids and other substances.
Q6. Which of the following are acidic and which are basic?
Lime water, Vinegar, Toothpaste, Stomach juices, Lemon juice, Baking soda solution, Milk of magnesia, Ammonia solution.
Ans: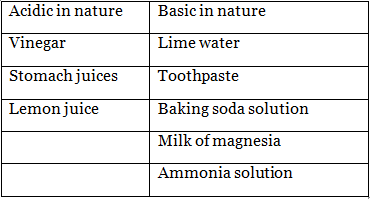
Q7. Write the effect of China rose petals on acidic and basic solutions.
Ans: The petals of the China rose have a unique characteristic that makes them useful in determining the nature of a solution, whether it is acidic or basic.
- When these petals are added to warm water, they create a light pink solution that can be used as an indicator in the lab.
- This indicator changes its color upon coming into contact with different types of solutions. In an acidic solution, the indicator turns a deep pink or magenta color, while in a basic solution, it transforms into a green color.
- This is due to the reaction between the indicator and the solution, which causes a color change based on the pH level of the solution.
Q8. Why are sodium bicarbonate and lemon juice used during indigestion?
Ans: Sodium bicarbonate and lemon juice are commonly used remedies for indigestion due to their properties.
- Sodium bicarbonate, also known as baking soda, has the ability to neutralize acidity. When we consume food, our stomach uses gastric acid to break down the food particles. However, if the acid level rises, it can lead to indigestion.
- In such situations, sodium bicarbonate comes to the rescue as it reduces the acid level in the stomach.
- On the other hand, lemon juice, which contains citric acid, aids digestion by reacting with the undigested food particles and helping to break them down. These two substances, therefore, play a crucial role in dealing with indigestion.
Q9. Explain two neutralisation reactions related to daily life situations.
Ans:
(i) Ant bite When an ant bite injects the acidic liquid (formic acid) into the skin, the effect of the acid can be neutralised by rubbing moist baking soda (sodium hydrogen carbonate) or calamine solution, which contains zinc carbonate.
(ii) Indigestion Our stomach contains hydrochloric acid. It helps us to digest food but too much of acid in the stomach causes indigestion. Sometimes, indigestion is painful. To relieve indigestion, we take an antacid such as milk of magnesia which contains magnesium hydroxide.
It neutralises the effect of excessive acid.
Q10. Name three types of salts. Give one example of each type of salt.
Ans: Salts are classified into three main types: neutral salts, acidic salts, and basic salts.
- Neutral salts are those formed by the complete neutralization of an acid with a base. Sodium chloride (NaCl) is a prime example of a neutral salt.
- Acidic salts, such as Ammonium chloride (NH4Cl), are formed when a base is not completely neutralized by an acid.
- Lastly, basic salts, like sodium hydrogen carbonate (NaHCO3), are produced when an acid is not completely neutralized by a base.
Q11. Calamine solution is applied on the skin when an ant bites. Give reason.
Ans. When an ant bites, it injects the acidic liquid (formic acid) into the skin. The effect of the acid can be neutralised by rubbing moist baking soda (sodium hydrogen carbonate) or calamine solution, which contains zinc carbonate.
Q12. Name three bases used in the laboratory with their formulae.
Ans: Bases which are mostly used in laboratory as below
- Sodium hydroxide (NaOH)
- Calcium hydroxide [Ca(OH)2]
- Ammonium hydroxide (NH4OH)
Q13. You are provided with three test tubes A, Sand Cas shown in figure with different liquids. What will you observe when you put
(a) a piece of blue litmus paper in each test tube?
(b) a piece of red litmus paper in each test tube?
(c) a few drops of phenolphthalein solution to each test tube?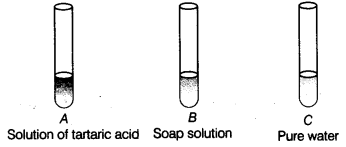
Ans:
Q14. Explain the terms acids and acidic.
Ans: The substances that taste sour are called acids, such as curd, lemon juice, orange juice, vinegar, etc. The chemical nature of these substances are acidic.
Q15. What are bases? What is their nature?
Ans: The substances that taste bitter and feel soapy on touching are called bases; e.g., lime water, baking soda, washing soda, etc. The nature of these substances are basic.
Q16. What are indicators?
Ans: Indicators are special type of substances that are used to test whether a substance is acidic or basic. They change their colour when added to a solution containing an acidic or a basic substance. For example, turmeric, China rose petals, litmus, etc., are naturally occurring indicators.
Q17. What is litmus?
Ans: Litmus is the most commonly used natural indicator. It is extracted from lichens. It has mauve (purple) colour in distilled water when added to an acidic solution, it turns red and when added to a basic solution, it turns blue. It may be in the form of a solution or in the form of strips of paper called litmus paper, which are generally red and blue.
Q18. What is neutralisation? Give an example of a neutralisation reaction.
Ans: The reaction between an acid and a base is called neutralisation. In this process, salt and water are formed with the evolution of heat.
Acid + Base ➝ Salt + Water + (heat is evolved)
Here is a reaction showing neutralisation.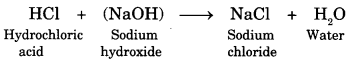
Q19. What is meant by acid rain?
Ans: The rain containing excess of acids is called acid rain. The rain becomes acidic because carbon dioxide, sulphur dioxide and nitrogen dioxide, which are released into the air as pollutants, dissolve in rain water to form carbonic acid, sulphuric acid and nitric acid respectively. Acid rain can cause damage to buildings, historical monuments, plants and animals.
Q20. What are the applications of neutralisation reaction in everyday life?
Ans: Neutralisation helps us in many ways in our everyday life. Some of the applications are:
- Indigestion: When we suffer from acidity we take antacid to get relief. Antacid neutralises the effect of excessive acid.
- Ant bite: When an ant bites, moist baking soda solution or calamine solution is rubbed which neutralises the effect of acid injected into the skin when an ant bites.
- Soil treatment: Plants do not grow well when the soil is too acidic or too basic. When the soil is too acidic, it is treated with bases like quick lime or slaked lime and when it is basic, organic matter is added to it. Organic matter releases acids which neutralise the basic nature of the soil.
- Factory wastes: The factory wastes contain acids, they are harmful for water bodies. To neutralise these wastes, basic substances are added to them.
Q21. Explain the process of neutralisation with the help of an activity.
Ans: When an acidic solution is mixed with a basic solution, both the solutions neutralise the effect of each other. The resulting solution is neither acidic nor basic. We can show the process of neutralisation with the help of an activity.
Fill one-fourth of a test tube with dilute hydrochloric acid. Note down its colour and also the colour of . phenolphthalein solution. Add 2-3 drops of the indicator to the acid. Shake the test tube gently. We observe that solution remains colourless. Add sodium hydroxide solution in the test tube drop by drop with continuous stirring till the pink colour just appears. Appearance of pink colour indicates that the neutralisation reaction has completed.
|
111 videos|286 docs|28 tests
|
FAQs on Class 7 Science Chapter 4 Question Answers - Acids, Base and Salts
| 1. What are some common examples of acids, bases, and salts? |  |
| 2. How are acids and bases different from each other? |  |
| 3. What is the importance of acids, bases, and salts in everyday life? |  |
| 4. How do acids and bases react with each other to form salts? |  |
| 5. What are some common uses of salts in various industries? |  |






















