Solved Examples: Some Basic Concepts of Chemistry- 2 | Physical Chemistry for NEET PDF Download
Q.1. Out of the following the largest number of atoms are contained in
(a) 11 g of CO2
(b) 4g of H2
(c) 5 g of NH3
(d) 8 g of SO2
Ans. (b)
Solution.
(a) CO2 = 11/44 = 0.25 mole
(b) 4/2 = 2 mole
(c) 5/17 = 2 × NA largest no. of atoms
(d) 8/64 = 1/8
Q.2. The percentage of nitrogen in urea, (NH2CONH2) is:
(a) 38.4
(b) 46.6
(c) 59.1
(d) 61.3
Ans. (b)
Solution.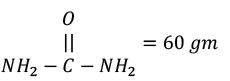
⇒ 60 gm urea = 28 gN
100 g 28 × 100/60 = 46.6%
Q.3. The empirical formula of a compound is CH2O, if vapour density is 90. Then the molecular formula is
(a) CH2O
(b) C2H4O2
(c) C3H6O3
(d) C6H12O6
Ans. (d)
Solution.
⇒ V. D × = mw
⇒ 90 × 2 = 180 gm
Empirical formula weight = 30 gm
Value of n = 180/30 = 6
C6H12O6
Q.4. An organic compound having carbon and hydrogen has 80% carbon. The empirical formula of the hydrocarbon is
(a) CH4
(b) CH3
(c) CH2
(d) CH
Ans. (b)
Solution.
C = 80%, h = 20%
%C = 80/12, %H = 20/1
= 6.66 = 20
= 6.66/6.66 = 20/6.66 = 3 {least Ratio}
1 : 3
C1H3 = CH3
Q.5. A compound contains 90% C and 10% H. The empirical formula of the compound is
(a) C8H10
(b) C15 H30
(c) C3H4
(d) C15H32
Ans. (c)
Solution.
%C = 90/12, %H = 10/1
= 7.5/7.5 = 10/7.5 = 1.33
= 1
C3H4
Q.6. An organic compound contains C = 50% and H = 9.25%. Its empirical formula is
(a) C3H6
(b) C3H7O2
(c) C2H4O
(d) C4H8O
Ans. (b)
Solution.
C% = 50/12 = 4.1666/2.546 = 1.636 × 2
%H = 9.25%/1 = 9.25/2.546 = 2.546/2.546 = 3.633 × 2
O% = 40.76/16 = 2.546/2.546 = 1 × 2
Ch3H7O2
Q.7. A metal M having an atomic weight of 197 yields a chloride containing 35.1% chlorine.
The empirical formula of the compound is
(a) MCl3
(b) MCl
(c) MCl2
(d) MCl4
Ans. (a)
Solution.
Eq. weight of metal chloride = % of metal/% chloride × 35.5 = 64.9/35.1 × 35.5 = 64.9
Eq = 197/VF
= 64.9 = 197/Vf = 197/64.9
VF = 3
M+3 Cl⊖
MCL3
Q.8. When 1 gram CaCO3 is dissolved in excess dilute acid the volume of CO2 evolved at STP will be
(a) One litre
(b) 224 ml
(c) 22.4 litre
(d) 2.24 litre
Ans. (b)
Solution.
CaCO3 ⟶ Cao + CO2
1 gm -------- 44 × 10-2 gm
44 g ----- 22.4 litres
44 × 10-2 g ------ = 0.224 litre = 224 ml
Q.9. 8 gram of sulphur is completely burnt in a large excess of oxygen, the volume in litres of SO2 formed as reduced to STP is
(a) 5.6
(b) 8.0
(c) 11.2
(d) 16.0
Ans. (a)
Solution.
S + O2 → SO2
32 g ------- 64 g = 1 mole = 22.4 liter
8g ----- 64 x 8/32 = 16 g
mole = 16/64 = 1/4 mole
5.6 litre value
Q.10. 0.01 mole of iodoform (CHI3) reacts with Ag powder to produce a gas whose volume at NTP is
(a) 224 ml
(b) 112 ml
(c) 336 ml
(d) 1120 ml
Ans. (b)
Solution.
2CHl3 + 6Ag ⟶ H − C ≡ C − H + 6 Agl
2 -------- 1 mole
10-2 --------- ?
10-2 × 1/2 = 0.5 × 10-2
= 5 × 10-3
∴ 1 mole ------ 22.4 literes
5 × 10-3 ------- 22.4 × 5 × 10-3
= 112 × 10-3
112 ml
Q.11. Molarity of liquid HCl with density equal to 1.17 g/ cc is
(a) 36.5
(b) 18.25
(c) 32.05
(d) 4.65
Ans. (c)
Solution.
d = M/v
1 cc = 1 ml
1000 ml = 1.17 × 1000 = 1170 g/36.5 = 32.05
Q.12. How many milliliters (mL) of 1 M H2SO4 solution are required to neutralize 10 mL of 1 M NaOH solution?
(a) 2.5 mL
(b) 5.0 mL
(c) 10.0 mL
(d) 20.0 mL
Ans. (b)
Solution.
Neutralization
Acid = Base (milliequivalent)
N1V1 = N2V2
⇒ 1 × V = 10 × 1 (N = m × VF)
V = 5ml
Q.13. The No. of moles of barium carbonate which contain 1.5 moles of oxygen atoms is
(a) 1.0 mole
(b) 1.5 mole
(c) 0.5 mole
(d) 2.0 mole
Ans. (c)
Solution.
Baco3 → 1 mole BaCo3
→ 1 mole Ba
→ 1 mole C
→ 3 mole (O)
3 mole oxygen - 1 mole BaCo3
1.5 mole oxygen - 1.5/3 = 0.5 mole BaC03
Q.14. A molecule of Haemoglobin contains 0.33% of iron by weight. The molecular weight of Haemoglobin is 67200. The number of iron atoms (At. wt. = 56) present in one molecule of Haemoglobin is
(a) 1
(b) 2
(c) 3
(d) 4
Ans. (d)
Solution.
Mass of Fe in 1 mole of Hb = ?
100 gm Hb – 0.33 gm Fe
1 mole(hb) = 67200g - 0.33 × 67200/100
= 221.76 gm
Mole of Fe = 221.76/56 = ≅ 3.9 ≅ 4
= 4NA atom of Fe
Na molecules of Hb – 4NA atom of Fe
1 mole - 4NA/NA
= 4 atoms of Fe
Q.15. 48 gram of Mg contains the same number of atoms as 160 gram of another element. The atomic mass of the element is
(a) 24
(b) 320
(c) 80
(d) 40
Ans. (c)
Solution.
mole of mg = 48/24 = 2 mole = 2 × NA
(A) = 160/2 = 80
Q.16. The number of moles of oxygen in one litre of air (21% oxygen by volume) at STP would be
(a) 0.186 mole
(b) 0.21 mole
(c) 2.10 mole
(d) 0.0093 mole
Ans. (d)
Solution.
In 100 ml air ---------- 21 ml oxygen
1000 ml air ----------- 21 × 1000/100 = 210 ml
22400 ml – 1 mole oxygen
210 ml - 210 × 1/22400 = 0.009375 mole oxygen
Q.17. What is correct for 10 g of CaCO3?
(a) It contains 1 g atom of carbon
(b) It contains 0.3 g atoms of oxygen
(c) It contains 12 g of calcium
(d) It refers to 0.1 g equivalent of CaCO3.
Ans. (b)
Solution.
10/100 = 0.1 mole CaCO3
1 mole CaCO3 →1 mole Ca
→1 mole C
→3 mole
∴ 0.1 mole CaCO3 → 0.1 Ca
→0.1C
→0.3(oxygen)
Q.18. If isotopic distribution of C-12 and C-14 is 98% and 2% respectively, then the number of C-14 atoms in 12 g of carbon is
(a) 1.032 × 1022
(b) 3.01 × 1022
(c) 5.88 × 1023
(d) 6.02 × 1023
Ans. (a)
Solution.
In 100 gram of carbon C-14 is=2 gram
12 gm = 2 × 12/100 = 0.24 gram
Mole of C-14 = 0.24/14 =o.017
Total no. of atoms C-14 = 0.017 × NA
= 0.0017 × 6.023 × 1023
= 0.102 × 1023
= 1.02 × 1022 atom
Q.19. The number of moles of water present in 100g of water of 90% purity is
(a) 3
(b) 18
(c) 5
(d) 5.5
Ans. (c)
Solution.
100% purity = 100/18 mole H2O
90% = 100 × 90/18 × 100
5 mole of H2O
Q.20. Which of the gases contains the same number of molecules as that of 16 grams of oxygen
(a) 16g of O3
(b) 32g of SO2
(c) 16g of SO2
(d) All
Ans. (b)
Solution.
16/16 = 1 mole NA atom = NA/2 = molecals
(a) 6/48 = 1/2 × NA
(b) 32/64 = 1/2
(c) 16/4 = 1/4
Q.21. The correct arrangement of the following in order of increasing mass is
(i) N2 molecule
(ii) oxygen atom
(iii) 1 Avogram
(iv) 1 × 10-10 gram atom of copper
(a) I > II > III > IV
(b) III < II < I < IV
(c) I > III > II > IV
(d) IV > I > II > III
Ans. (d)
Solution.
(a) N2 mole = 28 gm
(b) O atom = 16 gm
(c) 1/6 × 10 - 23 gm
(d) 63.5
1 mole of Cu = 63.5 gm
1 × 10-10 mol = 63.5 × 10-10 gm
Q.22. 0.5 mole of a gas (Mol. Wt. 80) occupies 11.2 litres at STP. The volume occupied by 0.25 mole of a lighter gas (Mol. Wt. 20) at STP will be
(a) 11.2 lit
(b) 5.6 lit
(c) 8 lit
(d) 22.4 litre
Ans. (b)
Solution.
0.5 mole - 11.2 litre
0.25 mole → 11.2 × 0.25/0.5 = 5.6 litre
Q.23. The molecular weight of an unknown substance is found to 24000. If it contains 0.2% Magnesium, then the number of Magnesium atoms that can be present in a molecule of it is
(a) 1
(b) 2
(c) 4
(d) 10
Ans. (b)
Solution.
100 gm-------2 gm Mg
2400--------2 × 2400/100 = 48 g
Mole of mg = 48/24 = 2 mole
Total atom of Mg = 2 × NA
NA molecules of unknown sub = 2NA
0.1 mole = 2 × NA/NA = 2
Q.24. The percentage of magnesium in chlorophyll is 2.68%. The number of magnesium atoms in 2 gram of chlorophyll is
(a) 1.34 × 1021
(b) 1.34 × 10–21
(c) 1.35 × 10–24
(d) 1.35 × 1024
Ans. (a)
Solution.
100 g – 2.68 g Mg
2g - 2.68 × 2/100 = weight of Mg
Mole= 2.68 × 2/100 × 24 = 0.2 × 10-2
Total atom of Mg = 0.22 × 10-2 × 6.023 × 1023
= 1.34 × 1021
Q.25. Ordinary water contains one part of heavy water per 6000 parts by weight. The number of heavy water molecules present in a drop of water of volume 0.01 ml is
(a) 2.5 × 1016
(b) 5 × 1017
(c) 5 × 1016
(d) 7.5 × 1016
Ans. (c)
Solution.
D2O = Mw = 20
Mole of D2O = 0.01/20 = 5 × 10-4 mole D2O
6000 parts ----5 × 10-4 mol D2O
1 part----- 5 × 10-4/6000 × NA
= 5 × 1016
Q.26. The number of molecules present in a drop of water. If its volume is 0.05 ml are
(a) 1.66 × 1021
(b) 1.60 × 1022
(c) 1.66 × 1023
(d) 1.60 × 1024
Ans. (a)
Solution.
H2O density = gm/ml =1 ml=1 gm
⇒ mole H2O = 0.05/18 × NA
= 0.05/18 × 6.023 × 1023
= 1.66 × 1021
Q.27. Which of the following will not have a mass of 10 g?
(a) 0.1 mol CaCO3
(b) 1.51 × 1023 Ca2+ ions
(c) 0.16 mol of Cl-ions
(d) 7.525 × 1022 Br atom.
Ans. (c)
Solution.
(a) CaCO3 = 0.1 mol = 10g
(b) NA Ca2+- 408 1.51 × 1023 - 1.51 × 1023/6 × 1023 = 1/4 × 40 = 10 g
(c) 1 mole Cl =35.5 0.16 = ? 0.16 × 35.5 = 5.68 gm × 2 ≅ 10
(d) 7.525 × Br atom
1 mole – 6.023× 1023
? --- 7.525 × 1022
⟹ 7.525 × 1022/6.2023× 1023 = 1.2 × 10-1
Q.28. x L of N2 at STP contains 3 × 1022 molecules. The number of molecules in x/2 L of ozone at STP will be
(a) 3 × 1022
(b) 1.5 × 1022
(c) 1.5 × 1021
(d) 1.5 × 1011
Ans. (b)
Solution.
xLN2 at stp → 3 x 1022 molecules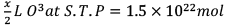
∴ volume occupied by gas at S.T.P doesn’t depend on nature of gas.
Q.29. 10 ml of a gaseous hydrocarbon combustion gives 40 ml of CO2 and 50 ml of H2O vapour under the same condition. The hydrocarbon is
(a) C4H6
(b) C6H10
(c) C4H8
(d) C4H10
Ans. (d)
Solution.
XHy + 02[x + y/4] C02 → + H2O
Volume of CO2 formed
10x = 40ml CO2
X = 4
X = c = 4
C4H10
volume of H2O
10 y/2 = 50ml H2O
y = 100/4 = 10
y = H = 1
OR
P.O.A.C method
P.O.A.C for carbon C atom
10x = 40
X = 4
C4H10
D.O.A.C for H atom
10y/2 = 50
y = 100/10
y = 10
Q.30. 15 ml hydrocarbon requires 45 ml of O2 for complete combustion and 30 ml of CO2 is formed. The formula of the hydrocarbon is
(a) C3H6
(b) C2H6
(c) C4H10
(d) C2H4
Ans. (d)
Solution.
CxHy + 02 → C02 + H2O
x + Y/4 x y
(1) volume of CO2 formed
15x = 30 ml of CO2
X = 2
(2) The volume of O2 required 15x + Y/4 = 45 ml of O2
⟹ 30+15Y/4 = 45
⟹ 120+15y/4 = 45
⟹ 120+15y = 180
⟹ 15y = 180-120
15 y = 60
Y = 60/15 = 4
C2H4
x = 2 y = 4
Q.31. Complete combustion of a sample of a hydrocarbon gives 0.66 2 grams of CO2 and 0.362 grams of H2O the formula of a compound is
(a) C3H8
(b) CH4
(c) C2H6
(d) C2H4
Ans. (a)
Solution.
hydrocarbon+ O2 → CO2 + H2O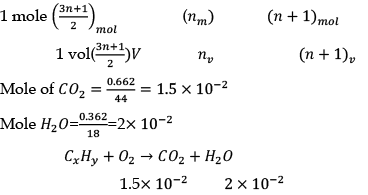
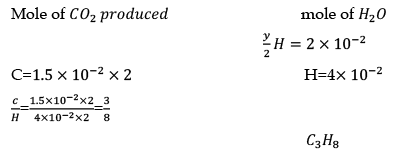
Q.32. The simplest formula of a compound containing 50% of element x (atomic weight = 10) and 50% of element y (atomic weight = 20) is
(a) xy
(b) x2y
(c) xy2
(d) x2y3
Ans. (b)
Solution.
%x = 50/10
x = 5/2.5
x = 2
y% = 50/20
y = 2.5/2,5
y = 1
x2y
Q.33. The empirical formula of an organic compound is CH. 6.023 × 1022 molecules of same organic compound weigh 7.8 g. The molecular formula is
(a) C2H2
(b) C6H6
(c) C2H4
(d) None
Ans. (b)
Solution.
empirical formula weight = 12+1 = 13g
6 × 1022− 7.8g
6 × 1023 - ?
= 7.8 × 6 × 1023/6 × 1022
= 78 gm
n = 78/13 = 6
CnHn = C6H6
Q.34. An organic compound contains C = 21.56%, H = 4.56% and Br = 73.36%. Its molecular weight is 109. Its molecular formula is
(a) C2H5Br
(b) C3H7Br
(c) C4H8Br
(d) C6H6Br
Ans. (a)
Solution.
C% = 21.56/12 = 1.7/0.917 = 1.853 ≅ 2
H% = 4.56/1 = 4.56/0917 = 4.97 ≅ 5
Br = 73.36/80 = 0.917/0.917 = 1
n = log/log = n
e.f = m.f
= C2H5Br
Q.35. 0.078 gram of hydrocarbon occupies 22.4 ml volume at STP. The empirical formula of hydrocarbon is CH. The molecular formula of hydrocarbon is
(a) C5H5
(b) C6H6
(c) C2H2
(d) C8H8
Ans. (b)
Solution.
22.4 ml--- 0.078 gm hydrocarbon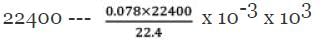
= 78 g
n = 78/13 = 6 CH = 13
12+1
C6H6
Q.36. Empirical formula of a compound is CH2. The mass of one litre of this organic gas is exactly equal to that of one litre of nitrogen. Therefore the molecular formula of the organic gas is
(a) C3H8
(b) C2H6
(c) C2H4
(d) C3H6
Ans. (c)
Solution.
e.f = CH2 12 + 2 = 14
n= 28/14 = 2
C2H4
Q.37. When 1.2 g of carbon is completely burnt in 6 litres of oxygen at STP, the remaining volume of oxygen is
(a) 3.76 lit
(b) 2.6 lit
(c) 5.8 lit
(d) 37.6 lit
Ans. (a)
Solution.
C + 1/2 O2 → (Incomplete combustion)
1C → (1 mole oxygen)
0.1C → 0.1 × 1 = 0.1 mole of oxygen
⟹ 22.4 litre → 1 mole O2
1 C --------- 22.4 litre
0.1 ---------- 22.4 litre
6 − 2.24 = 3.76 liter
Q.38. 0.5 mole of H2SO4 is mixed with 0.2 mole of Ca (OH)2. The maximum number of moles of CaSO4 formed is
(a) 0.5
(b) 0.2
(c) 0.4
(d) 0.25
Ans. (b)
Solution.
H2SO4 + Ca(OH)2 → CaSO4 + 2H2O
d = 0 0.5 0.2 0
t = ? 0.5 - x 0.2 - x x
x = 0.2
LR
L.R = 0.5/1
L.R = 0.2/1 = 0.2 = x
nx = aa
1 × = 0.2 × 100/100
x = 0.2
Q.39. 70 gram of a sample of magnesite on treatment with excess of HCl gave 11.2 litre of CO2 at STP. The percentage purify of the sample is
(a) 80
(b) 70
(c) 60
(d) 50
Ans. (d)
Solution.
Magnetic MgCO3+ Fe]=140gm
Fe + MgCO3 → MgCl2 + H2CO3
↓
H2O = CO2
1 mole----------1CO2
1 CO2------------1 mol MgCO3
0.5--------------0.5 mol
70/140 × 100
% purity = 50%
Q.40. When 100 gram of ethylene polymerises to polythene according to the equation nCH2 = CH2 → (CH2 - CH2)n - the weight of polythene produced will be
(a) n/2 gram
(b) 100 gram
(c) 100/n gram
(d) 100n gram
Ans. (b)
Solution.
nCH2 = CH2 → (-CH2 - CH2 -)n
100g 100g
n28gm -------- nx28g
Q.41. Air contains 20% by volume of oxygen. The volume of air required for the complete combustion of one litre of methane under the same conditions is
(a) 2 litre
(b) 4 litre
(c) 10 litre
(d) 0.4 litre
Ans. (c)
Solution.
CH4 +202 → CO2 +2H2O
1 litre 2 litre
2 liter O2 → 1 litre CH4
20 litre ---- 20/2 = 10 litre CH4
Q.42. The hydrated Na2SO4nH2O undergoes 56% loss in weight on heating and become anhydrous. The value of n will be
(a) 5
(b) 3
(c) 7
(d) 10
Ans. (d)
Solution.
Na2SO4H2O → Na2SO4+ xH2O
142 + 18x = 56/100%
Lose in weigh due to H2O = 18x/142 + 18x = 56/100
⟹ 1800x = 142 × 56 + 18x × 56
⟹ 1800x = 7952 + 1000x
⟹ 792x = 795x
x = 10.004
x = 10
Q.43. 1.25 g of a solid dibasic acid is completely neutralized by 25 mL of 0.25 molar Ba (OH)2 solution . The molecular mass of the acid is
(a) 100
(b) 150
(c) 120
(d) 200
Ans. (d)
Solution.
Neutralisation rxn
Solid dibasic acid =
N1V1 = N2V2
1000 × w/e = N1V1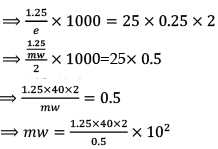
mw = 160 × 1.25
mw = 200
Q.44. 0.126 g of acid requires 20 ml of 0.1 N NaOH for complete neutralization. The equivalent mass of the acid is
(a) 45
(b) 53
(c) 40
(d) 63
Ans. (d)
Solution.
w/e × 1000 = 20 × 0.1
⟹ 0.126/E × 1000 = 2
E = 0.126 × 1000/2
E = 63
Q.45. The mole fraction of the solute in one mole aqueous solution is
(a) 0.009
(b) 0.018
(c) 0.027
(d) 0.036
Ans. (b)
Solution.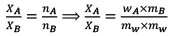


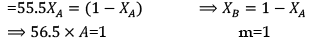
XA = 1/56.5 = 0.18
Q.46. How many grams of phosphoric acid would be needed to neutralize 100 g of magnesium hydroxide?
(a) 66.7 g
(b) 252 g
(c) 112 g
(d) 168 g
Ans. (c)
Solution.
⇒ w/E1 × 1000 = w2/E2 × 1000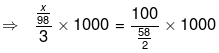
⇒ 3x/98 = 200/58
⇒ x = 200 × 98 / 58 × 3 = 19600/174
= 112g
Q.47. Normality of solution of FeSO4. 7H2O containing 5.56 g / 200 mL which converts to ferric from in a reaction is (Fe = 56, s = 32, O = 16, H = 1)
(a) 1
(b) 0.1
(c) 0.01
(d) 10
Ans. (b)
Solution.
Molarity of FeSO47H2O
156 + 126 = 278g mw
Mole = 5.56/278g mol
200-----5.56/278
1000------------?
5.56 × 1000/278 × 200 × 10-2
= 20/200 = 0.1
Fe2+ → Fe3+ VF----1
N = MXVF
N = 0.1 × 1
N = 0.1N
Q.48. 100 g of a sample of HCl solution of relative density 1.17 contains 31.2 g of HCl. What volume of this HCl solution will be required to neutralize exactly 5 litres of N/20 KOH solution?
(a) 25 ml
(b) 29.2 ml
(c) 34.2 ml
(d) 250 ml
Ans. (a)
Solution.
D = m/v
1.74 × g/m =100/V
V = 100/1.17
V = 85.4m volume of solution
⟹ 85.4 - 31.2/36.5 = 0.84
1000ml -31.2 × 1000/36.5 × 85.4
M = 10m
N= m × VF
N=10N----1
Molarity of HCl solution = 10m
Normality = molarity × VF
= 10 ×1
= 10N
N1V1 = N2V2
⟹ 10 × Vlitre = 5/10
Vlitre = 5/100 = 0.025 litre
= 25 ml
Q.49. 300 ml of 1 M HCI and 100 ml of 1 M NaOH are mixed. The chloride ion concentration in the resulting solution is
(a) 1 M
(b) 0.5 M
(c) 0.75 M
(d) 0.25 M
Ans. (c)
Solution.
HCL ⇋ H+ + Cl-
0.3 0.3 0.3
NaOH ⇋ Na+ + OH
0.1 0.1 0.1
Cl- = 0.3/0.4 = 0.75M
Cl- = c0ncentration of Cl-1/total volume
Q.50. 200 ml of 1 M H2SO4, 300 ml 3 M HCI and 100 ml of 2 M HCI is mixed and made up to 1 litre. The proton concentration in the resulting solution is
(a) 1.25 M
(b) 1.5 M
(c) 2.5 M
(d) 0.75 M
Ans. (b)
Solution.
All are acids so it shows additive properties
H+ = (N1V1+N2V2+N3V3)/(V1+V2+V3)
400+900+200/1000 = 1500/1000 = 1.5
Q.51. The volume of 0.25 M NaOH to be added to 250 mL of 0.15 M NaOH so that the resultant solution is 0.2 M would be
(a) 250 mL
(b) 350 mL
(c) 450 mL
(d) 550 mL
Ans: a
Solution: Let V be the volume of 0.25 M NaOH solution. Total amount of NaOH after mixing the two solutions is n = V (0.25 mol L–1) + (0.25 L) (0.15 mol L–1)
Total volume of the solution = V + 0.25 L
Molarity of the resultant solution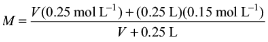
Equating this to 0.2 M, we get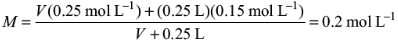
Solving for V, we get V = 0.25 L = 250 mL
Q.52. The mass of H2O2 that is completely oxidized by 30.2 g of KMnO4 (molar mass = 158 g mol–1) in acidic medium is
(a) 12 g
(b) 15 g
(c) 17 g
(d) 1 g
Ans: c
Solution: The reaction is 2KMnO4 + 5H2O2 + 6H+ → 2Mn2+ + 5O2 + 8H2O + 2K+
2 x 151 g of KMnO4 reacts completely with 5 x 34 g of H2O2
30.2 g of KMnO4 reacts completely with 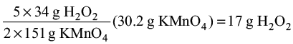
|
117 videos|226 docs|237 tests
|
FAQs on Solved Examples: Some Basic Concepts of Chemistry- 2 - Physical Chemistry for NEET
| 1. What are the basic concepts of chemistry? |  |
| 2. How are elements different from compounds? |  |
| 3. What is the significance of atomic structure in chemistry? |  |
| 4. How do chemical reactions occur? |  |
| 5. What is stoichiometry and its importance in chemistry? |  |
















