Class 10 Exam > Class 10 Notes > Chemistry Class 10 ICSE > Mind Map: Study of Compounds - Ammonia and Nitric Acid
Mind Map: Study of Compounds - Ammonia and Nitric Acid | Chemistry Class 10 ICSE PDF Download
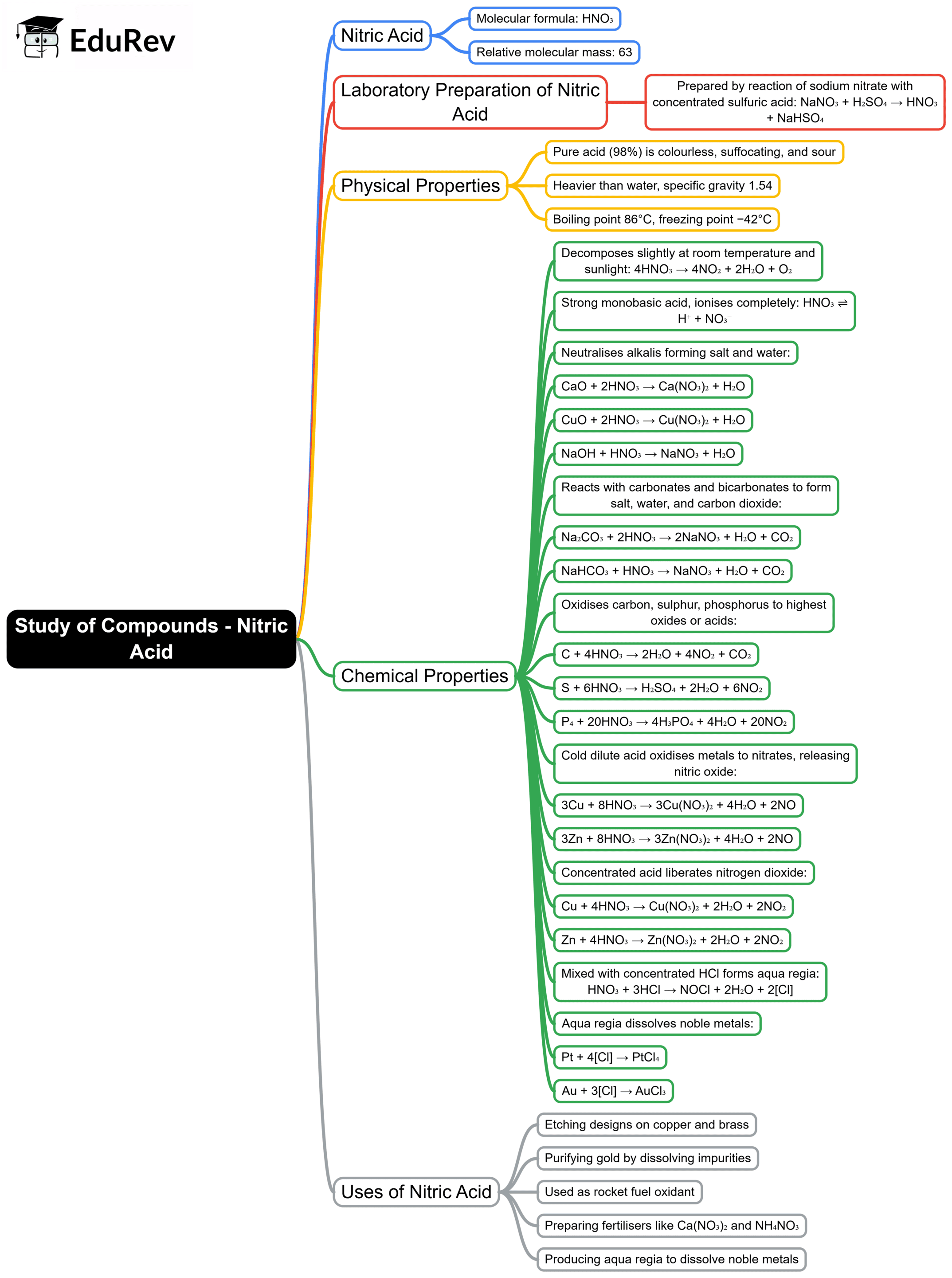
The document Mind Map: Study of Compounds - Ammonia and Nitric Acid | Chemistry Class 10 ICSE is a part of the Class 10 Course Chemistry Class 10 ICSE.
All you need of Class 10 at this link: Class 10
|
39 videos|85 docs|14 tests
|
FAQs on Mind Map: Study of Compounds - Ammonia and Nitric Acid - Chemistry Class 10 ICSE
| 1. What is the chemical formula of ammonia and how is it produced? |  |
Ans. The chemical formula of ammonia is NH₃. It is commonly produced through the Haber process, where nitrogen (N₂) from the air reacts with hydrogen (H₂) under high temperature and pressure in the presence of a catalyst.
| 2. What are the uses of nitric acid in various industries? |  |
Ans. Nitric acid (HNO₃) is widely used in fertilizers, explosives, and the production of plastics. It is also important in metal processing and as a reagent in laboratories for various chemical reactions.
| 3. What are the physical properties of ammonia and nitric acid? |  |
Ans. Ammonia is a colorless gas with a pungent smell, and it is highly soluble in water. Nitric acid is a yellowish liquid with a strong, acrid odor. It is corrosive and can form toxic fumes when heated.
| 4. How do ammonia and nitric acid react with each other? |  |
Ans. Ammonia reacts with nitric acid to form ammonium nitrate (NH₄NO₃), which is a common fertilizer. The reaction is exothermic and can be represented by the equation: NH₃ + HNO₃ → NH₄NO₃.
| 5. What safety precautions should be taken when handling ammonia and nitric acid? |  |
Ans. When handling ammonia, it is important to work in a well-ventilated area and use protective equipment such as gloves and goggles due to its toxicity and irritating properties. For nitric acid, similar precautions are necessary, as it is corrosive and can cause severe burns. Always store these chemicals properly and follow safety guidelines.
Related Searches





















