NCERT Exemplar: Biomolecules | Biology Class 11 - NEET PDF Download
MULTIPLE CHOICE QUESTIONS
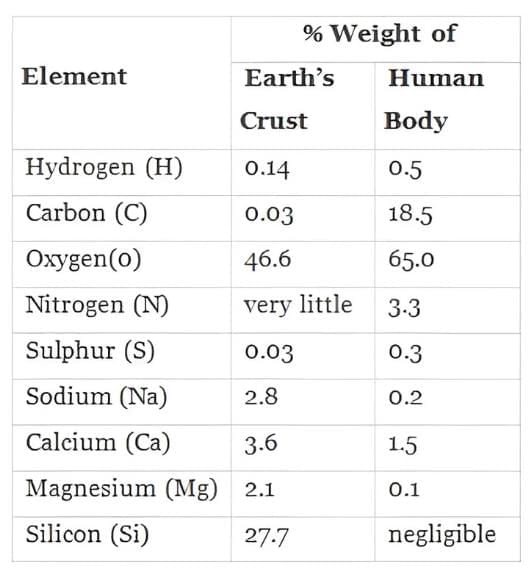
Q.1. It is said that elemental composition of living organisms and that of inanimate objects (like earth’s crust) are similar in the sense that all the major elements are present in both. Then what would be the difference between these two groups? Choose a correct answer from among the following:
(a) Living organisms have more gold in them than inanimate objects
(b) Living organisms have more water in their body than inanimate objects
(c) Living organisms have more carbon, oxygen and hydrogen per unit mass than inanimate objects.
(d) Living organisms have more calcium in them than inanimate objects.
Ans. (c)
Solution.
- Living organisms have a higher relative abundance of carbon, oxygen, and hydrogen compared to inanimate objects.
- This difference is essential for various biological processes.
Q.2. Many elements are found in living organisms either free or in the form of compounds. Which of the following is not found in living organisms?
(a) Silicon
(b) Magnesium
(c) Iron
(d) Sodium
Ans. (a)
Solution. Among the elements listed:
- Magnesium is vital for many metabolic functions.
- Iron is essential for oxygen transport in the blood.
- Sodium plays a key role in nerve function and fluid balance.
- Silicon, however, is not typically found in living organisms.
Thus, the correct answer is (a) Silicon.
Q.3. Aminoacids, have both an amino group and a carboxyl group in their structure. Which one of the following is an amino acid?
(a) Formic acid
(b) Glycerol
(c) Glycolic Acid
(d) Glycine
Ans. (d)
Solution.
Glycine is an amino acid (which have both an amino group and a carboxyl group in their structure).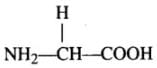
Q.4. An amino acid, under certain conditions have both positive and negative charges simultaneously in the same molecule. Such a form of amino acid is called
(a) Acidic form
(b) Basic form
(c) Aromatic form
(d) Zwitterionic form
Ans. (d)
Solution.
The term ‘zwitterion’ comes from a German word which means a neutral molecule with positive and negative charges.
Q.5. Sugars are technically called carbohydrates, referring to the fact that their formulae are only multiple of C(H2O). Hexoses therefore have six carbons, twelve hydrogens and six oxygen atoms. Glucose is a hexose.
Choose from among the following another hexose.
(a) Fructose
(b) Erythrose
(c) Ribulose
(d) Ribose
Ans. (a)
Solution. Fructose is another example of a hexose. It has the same molecular formula as glucose, which is: 6 carbon atoms, 12 hydrogen atoms and 6 oxygen atoms. Thus, fructose is classified as a hexose sugar.
Q.6. When you take cells or tissue pieces and grind them with an acid in a mortar and pestle, all the small biomolecules dissolve in the acid. Proteins, polysaccharides and nucleic acids are insoluble in mineral acid and get precipitated. The acid-soluble compounds include amino acids, nucleosides, small sugars, etc. When one adds a phosphate group to a nucleoside, one gets another acid-soluble biomolecule called
(a) Nitrogen base
(b) Adenine
(c) Sugar phosphate
(d) Nucleotide
Ans. (d)
Solution.
Nucleotide = base + sugar + phosphate
Q.7. When we homogenise any tissue in an acid, the acid-soluble pool represents
(a) Cytoplasm
(b) Cell membrane
(c) Nucleus
(d) Mitochondria
Ans. (a)
Solution. The acid-soluble pool mainly consists of the contents found in the cytoplasm of the tissue. This pool includes:
- Small organic compounds
- Water-soluble molecules
- Various metabolites
In contrast, the acid-insoluble fraction contains larger macromolecules, such as:
- Proteins
- Nucleic acids
- Polysaccharides
- Some lipids
Q.8. The most abundant component of living organisms is
(a) Protein
(b) Water
(c) Sugar
(d) Nucleic acid
Ans. (b)
Solution.
The most abundant component of a cell is water.
| Component | % of the total Cellular Mass |
| Water | 70-90 |
| Proteins | 10-15 |
| Nucleic acids | 5-7 |
| Carbohydrates | 3 |
| Lipids | 2 |
| Ions | 1 |
Q.9. A homopolymer has only one type of building block called monomer repeated ‘n’ number of times. A heteropolymer has more than one type of monomer. Proteins are heteropolymers usually made of
(a) 20 types of monomers
(b) 40 types of monomers
(c) 30 types of monomers
(d) only one type of monomer
Ans. (a)
Solution. A homopolymer consists of one type of building block, known as a monomer, repeated multiple times. In contrast, a heteropolymer contains more than one type of monomer. Proteins are classified as heteropolymers and are primarily composed of:
- 20 types of monomers (amino acids)
- 40 types of monomers
- 30 types of monomers
- Only one type of monomer
Therefore, the correct answer is (a) 20 types of monomers.
Q.10. Proteins perform many physiological functions. For example, some functions as enzymes. Which of the following represents an additional function that some proteins discharge?
(a) Antibiotics
(b) Pigment conferring colour to skin
(c) Pigments making the colours of flowers
(d) Hormones
Ans. (d)
Solution. Hormones are proteins that act as chemical messengers in the body. They help regulate various physiological processes, such as:
- Growth and development
- Metabolism
- Reproductive functions
Other functions of proteins include: Enzymatic activity, Immune response (e.g., antibodies) and Transport of molecules (e.g., haemoglobin)
Q.11. Glycogen is a homopolymer made of
(a) Glucose units
(b) Galactose units
(c) Ribose units
(d) Amino acids
Ans. (a)
Solution.
Glycogen is a homopolymer made of glucose units.
Q.12. The number of ‘ends’ in a glycogen molecule would be
(a) Equal to the number of branches plus one
(b) Equal to the number of branch points
(c) One
(d) Two, one on the left side and another on the right side
Ans. (a)
Solution.
- Glycogen is a multi-branched polysaccharide composed of glucose units. It is often referred to as "animal starch" due to its structural similarity to starch.
- Glycogen has two main types of linkages: - Alpha 1,4 linkages: These are found in the straight chains of glucose units - Alpha 1,6 linkages: These are found at the branching points.
- Each branch in the glycogen molecule introduces a new end. Therefore, the number of ends in glycogen can be calculated based on the number of branches.
- The total number of ends in a glycogen molecule can be expressed as:
- - Number of non-reducing ends = Number of branches + 1 (the +1 accounts for the main chain end).
Q.13. The primary structure of a protein molecule has
(a) Two ends
(b) One end
(c) Three ends
(d) No ends
Ans. (a)
Solution.
The primary structure of a protein molecule has two ends.
A protein is imagined as a line, the left end represented by the first amino acid and the right end is represented by the last amino acid. The first amino acid is also called as N-terminal amino acid. The last amino acid is called the C-terminal amino acid.
Q.14. Enzymes are biocatalysts. They catalyse biochemical reactions. In general, they reduce the activation energy of reactions. Many physicochemical processes are enzyme-mediated. Which of the following reactions is not enzyme-mediated in a biological system?
(a) Dissolving CO2 in water
(b) Untwining the two strands of DNA
(c) Hydrolysis of sucrose
(d) Formation of a peptide bond
Ans. (a)
Solution.
Dissolving CO2 in water is a physical process.
VERY SHORT ANSWER TYPE QUESTIONS
Q.1. Medicines are either man-made (i.e., synthetic) or obtained from living organisms like plants, bacteria, animals etc. and hence the latter are called natural products. Sometimes, natural products are chemically altered by man to reduce toxicity or side effects. Write against each of the following whether they were initially obtained as a natural product or as a synthetic chemical.
(a) Penicillin _________________
(b) Sulfonamide ________________
(c) Vitamin C __________________
(d) Growth Hormone ________________
Ans. (a) Penicillin: Natural product
(b) Sulfonamide: Synthetic chemical
(c) Vitamin C: Natural product
(d) Growth Hormone: Natural product
Q.2. Select an appropriate chemical bond among ester bond, glycosidic bond, peptide bond and hydrogen bond and write against each of the following.
(a) Polysaccharide ____________
(b) Protein ____________
(c) Fat ____________
(d) Water ____________
Ans.
(a) Polysaccharide: Glycosidic bond
(b) Protein: Peptide bond
(c) Fat: Ester bond
(d) Water: Hydrogen bond
Q.3. Write the name of any one amino acid, sugar, nucleotide and fatty acid.
Ans. Glycine (amino acid), Ribose (sugar), Cytidylic acid (nucleotide) and Arachidonic acid (fatty acid).
Q.4. Reaction given below is catalysed by oxidoreductase between two substrates A and A’, complete the reaction.
A reduced + A’ oxidised →
Ans: A reduced + A’ oxidised → A oxidised + A’ reduced
Q.5. How are prosthetic groups different from cofactors?
Ans. Prosthetic groups are organic compounds and are distinguished from other cofactors in that they are tightly bound to the apoenzyme. For example, in peroxidase and catalase, which catalyse the breakdown of hydrogen peroxide to water and oxygen, haem is the prosthetic group and it is a part of the active site of the enzyme. A cofactor may be organic or inorganic (metal ions).
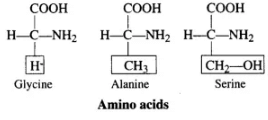
Q.6. Glycine and Alanine are different with respect to one substituent on the α -carbon. What are the other common substituent groups?
Ans. The R-group in proteinaceous amino acids can vary significantly. Here are some examples:
- Glycine: Contains a hydrogen atom as its R-group.
- Alanine: Features a methyl group (-CH3) as its R-group.
- Serine: Has a hydroxy methyl group (-CH2OH) as its R-group.
These variations contribute to the diverse properties and functions of amino acids in proteins.
Q.7. Starch, Cellulose, Glycogen, and Chitin are polysaccharides found among the following. Choose the one appropriate and write against each.
Cotton fibre ______________
Exoskeleton of cockroach ______________
Liver ______________
Peeled potato ______________
Ans.Cotton fibre: Cellulose
Exoskeleton of Cockroach: Chitin
Liver: Glycogen
Peeled potato: Starch
SHORT ANSWER TYPE QUESTIONS
Q.1. Enzymes are proteins. Proteins are long chains of amino acids linked to each other by peptide bonds. Amino acids have many functional groups in their structure. These functional groups are, many of them at least, ionisable. As they are weak acids and bases in chemical nature, this ionisation is influenced by pH of the solution. For many enzymes, activity is influenced by the surrounding pH. This is depicted in the curve below, explained brieflyAns. This graph illustrates a curve that rises and then falls, indicating that an enzyme has optimum activity at a specific pH level. Key points include:
- Below the optimum pH, the enzyme's activity is at its lowest.
- As the pH increases beyond the optimum, the enzyme's activity decreases again.
Thus, the surrounding pH significantly influences enzyme activity.
Q.2. Is rubber a primary metabolite or a secondary metabolite? Write four sentences about rubber.
Ans. Rubber is a secondary metabolite. Metabolites which do not have any identifiable function in the host organism are called secondary metabolites. Rubber does not have any known function for the plant, and hence it is called a secondary metabolite. However, rubber has certain economic significance for human beings. Rubber is used for making a variety of useful items, like tyres, erasers, toys, insulation layers, gloves, etc.
Q.3. Schematically represent primary, secondary and tertiary structures of a hypothetical polymer, say for example a protein.
Ans. The following diagrams show the primary, secondary and tertiary structures of protein: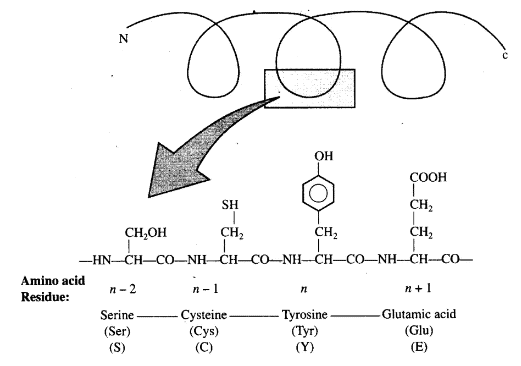
- Primary Structure: This is the sequence of amino acids in the protein chain. The left end is the N-terminal, and the right end is the C-terminal.
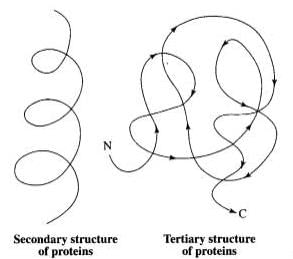
- Secondary Structure: This structure includes formations such as alpha-helices and beta-pleated sheets, which arise from hydrogen bonding between amino acids.
- Tertiary Structure: This is the overall 3D shape of the protein, formed by the folding of the protein chain upon itself.
Q.4. Nucleic acids exhibit secondary structure. Justify with an example.
Ans. Nucleic acids exhibit a variety of secondary structures. One prominent example is the Watson–Crick model of DNA, which describes its structure as a double helix. Key features include:
- The two strands of DNA are antiparallel, meaning they run in opposite directions.
- The backbone consists of a sugar-phosphate chain.
- The nitrogen bases (adenine, thymine, guanine, and cytosine) are oriented perpendicular to the backbone and face inward.
- Adenine (A) pairs with thymine (T) and guanine (G) pairs with cytosine (C) through hydrogen bonds: two bonds between A and T, and three between G and C.
- The structure resembles a helical staircase, with each step represented by a pair of bases.
- Each turn of the helix consists of about ten base pairs, with a pitch of 34 Å and a rise of 3.4 Å per base pair.
This specific form of DNA, characterised by these features, is known as B-DNA.
Q.5. Comment on the statement “living state is a non-equilibrium steady state to be able to perform work”.
Ans. The essence of biological systems is that all living organisms maintain a steady state characterised by specific concentrations of biomolecules. These molecules are constantly in metabolic flux. In nature, processes tend to move towards equilibrium, but living organisms exist in a non-equilibrium state. Key points to consider:
- Systems at equilibrium cannot perform work.
- Living organisms must continuously work to avoid reaching equilibrium.
- This non-equilibrium state is essential for performing biological work.
- Energy input is crucial to maintain this state.
Metabolism plays a vital role in producing energy, making it synonymous with the living state. Without metabolism, life cannot exist.
LONG ANSWER TYPE QUESTIONS
Q.1. Formation of the enzyme-substrate complex (ES) is the first step in catalysed reactions. Describe the other steps till the formation of the product.
Ans. The catalytic cycle of an enzyme action can be described in the following steps:
- Substrate binding: The substrate binds to the active site of the enzyme, fitting snugly into it.
- Induced fit: The binding of the substrate causes the enzyme to change shape, creating a tighter fit around the substrate.
- Formation of enzyme-product complex: The active site, now close to the substrate, breaks its chemical bonds, forming a new enzyme-product complex.
- Product release: The enzyme releases the products of the reaction, returning to its original state and ready to bind with another substrate molecule.
This process highlights the importance of the enzyme-substrate complex in facilitating chemical reactions.
Q.2. What are the different classes of enzymes? Explain any two with the type of reaction they catalyse.
Ans. Enzymes are divided into six classes. Each class is further subdivided into 4 to 13 sub-classes. The following are the six classes of enzymes:
(a) Oxidoreductase/Dehydrogenase: Enzymes which catalyse oxidoreduction between two substrates are called oxidoreductases. The following is an example of an oxidoreductase reaction:
S reduced + S’ oxidized → S oxidized + S’ reduced
(b) Transferase: Enzymes which facilitate the transfer of a group (other than hydrogen) between two substrates are called transferases. The following example shows a transferase reaction:
S-G+S’ → S+S’-G
(c) Hydrolase: Enzymes catalysing the hydrolysis of ester, ether, peptide, glycosidic, C-C, C-halide or P-N bonds.
(d) Lysase: Enzymes that catalyse the removal of groups from substrates by mechanisms other than hydrolysis, leaving double bonds.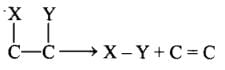 (e) Isomerase: Includes all enzymes catalysing the interconversion of optical, geometric or positional isomers.
(e) Isomerase: Includes all enzymes catalysing the interconversion of optical, geometric or positional isomers.
(f) Ligase: Enzymes catalysing the linking together of 2 compounds. Example: Enzymes which catalyse joining of C-O, C-S, C-N, P-O etc. bonds.
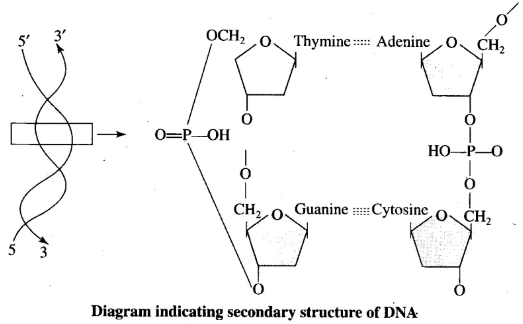
Q.3. Nucleic acids exhibit secondary structure. Describe through the Watson-Crick Model.
Ans. Nucleic acids exhibit various secondary structures, one of which is the Watson-Crick model for DNA. This model describes DNA as a double helix with the following features:
- The two strands of polynucleotides are antiparallel, meaning they run in opposite directions.
- The backbone consists of a sugar-phosphate chain.
- The nitrogen bases extend perpendicularly from the backbone and face inward.
- Adenine (A) pairs with thymine (T) and guanine (G) pairs with cytosine (C) through specific base pairing.
- A and T are connected by two hydrogen bonds, while G and C are connected by three hydrogen bonds.
- The structure resembles a helical staircase, where each step is a base pair.
- Each complete turn of the helix consists of ten base pairs and turns by 36 degrees.
- The pitch of the helix is 34 Å, and the rise per base pair is 3.4 Å.
This form of DNA, characterised by the features above, is known as B-DNA.
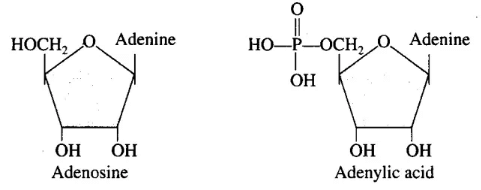
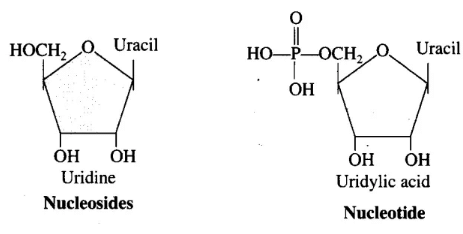
Q.4. What is the difference between a nucleotide and a nucleoside? Give two examples of each with their structure.
Ans. Living organisms have a number of carbon compounds in which heterocyclic rings can be found. Some of these are nitrogen bases—adenine, guanine, cytosine, uracil and thymine. When found attached to a sugar, they are called nucleosides. If a phosphate group is also found esterified to the sugar, they are called nucleotides. Adenosine, guanosine, thymidine, uridine and cytidine are nucleosides. Adenylic acid, thymidylic acid, guanylic acid, uridylic acid and cytidylic acid are nucleotides.
Nucleotides and nucleosides are both essential components of nucleic acids, but they differ in structure:
- Nucleosides consist of:
- A nitrogenous base (e.g., adenine, guanine)
- A sugar (either ribose or deoxyribose)
- Nucleotides include:
- A nitrogenous base
- A sugar
- A phosphate group
Examples include:
- Nucleosides:
- Adenosine
- Guanosine
- Nucleotides:
- Adenylic acid
- Guanylic acid
Q.5. Describe various forms of lipids with a few examples.
Ans. Lipids are generally water-insoluble. They could be simple fatty acids. A fatty acid has a carboxyl group attached to an R-group. The R-group could be a methyl (-CH3), or ethyl (—C2H5) or a higher number of CH2 groups (1 carbon to 19 carbons). For example, palmitic acid has 16 carbons, including a carboxyl carbon. Arachidonic acid has 20 carbon atoms, including a carboxyl carbon. Fatty acids could be saturated (without double bonds) or unsaturated (with one or more C=C double bonds). Another simple lipid is glycerol, which is trihydroxypropane.
- Many lipids have both glycerol and fatty acids. Here, the fatty acids are found esterified with glycerol. They can be then monoglycerides, diglycerides and triglycerides. These are also called fats and oils based on melting point. Oils have lower melting points (Example: gingerly oil) and hence remain as oil in winter.
- Some lipids have phosphorus and a phosphorylated organic compound in them. These are phospholipids. They are found in cell membranes. Lecithin is one example. Some tissues, especially the neural tissues, have lipids with more complex structures.
|
183 videos|524 docs|136 tests
|
FAQs on NCERT Exemplar: Biomolecules - Biology Class 11 - NEET
| 1. What are biomolecules and why are they important for living organisms? |  |
| 2. What are the main types of biomolecules and their functions? |  |
| 3. How do enzymes, as proteins, function in biological systems? |  |
| 4. What is the significance of nucleic acids in heredity? |  |
| 5. How do lipids differ from carbohydrates and proteins in terms of structure and function? |  |

















