NCERT Exemplar: Chemical Kinetics | Chemistry Class 12 - NEET PDF Download
MULTIPLE CHOICE QUESTIONS
Q.1. The role of a catalyst is to change ___________.
(i) Gibbs energy of reaction.
(ii) Enthalpy of reaction.
(iii) Activation energy of reaction.
(iv) Equilibrium constant.
Ans. (iii)
Solution.
A catalyst lowers the activation energy of a reaction.
Q.2. In the presence of a catalyst, the heat evolved or absorbed during the reaction ___________.
(i) Increases.
(ii) Decreases.
(iii) Remains unchanged.
(iv) May increase or decrease.
Ans. (iii)
Solution.
There is no effect on heat evolved or absorbed during the reaction in the presence of a catalyst since it does not participate in the reaction.
Q.3. Activation energy of a chemical reaction can be determined by ________.
(i) Determining the rate constant at standard temperature.
(ii) Determining the rate constants at two temperatures.
(iii) Determining probability of collision.
(iv) Using catalyst.
Ans. (ii)
Solution.
Activation energy of a chemical reaction is related to rate constant of a reaction at two different temperatures i.e.,K1 and K2 respectively.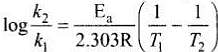
where, Ea = activation energy
T2 = higher temperature
T1 = lower temperature
k1= rate constant at tem perature T1,
k2 = rate constant at temperature T2
This equation is known as Arrhenius equation.
Q.4. Consider Fig. 4.1 and mark the correct option.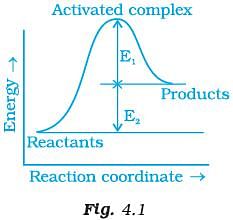 (i) Activation energy of forward reaction is E1 + E2 and product is less stable than reactant.
(i) Activation energy of forward reaction is E1 + E2 and product is less stable than reactant.
(ii) Activation energy of forward reaction is E1 + E2 and product is more stable than reactant.
(iii) Activation energy of both forward and backward reaction is E1 + E2 and reactant is more stable than product.
(iv) Activation energy of backward reaction is E1 and product is more stable than reactant.
Ans. (i)
Solution.
Ea(forward) = E1 + E2
Since energy of reactants is less than products and the product is less stable than the reactant.
Q.5. Consider a first order gas phase decomposition reaction given below :
A(g) → B(g) + C(g)
The initial pressure of the system before decomposition of A was pi. After lapse of time ‘t’, total pressure of the system increased by x units and became ‘pt’ The rate constant k for the reaction is given as _________.
(i)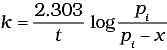
(ii)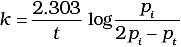
(iii)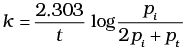
(iv)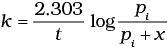
Ans. (ii)
Solution.
Consider a first order gas phase decomposition reaction:
A(g) → B(g) + C(g)
The initial pressure of the system before decomposition of A was Pi.
After lapse of time (t), total pressure of the system increased by x units and became 'Pt'
In other words, the pressure of A decreased by x atom.
A(g) → B(g)→ + C(g)
Initial pressure: Pi atm 0 0
Pressure after time. t: (Pi, - x) atm x atm x atm
Pt = ( Pi - x) + x + x = Pi +x atm
x = Pt - Pi
Pressure of A after time t, PA = Pi - x
= Pi - Pt + Pi
= 2Pi - Pt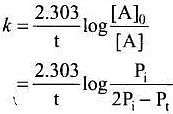
Q.6. According to Arrhenius equation rate constant k is equal to Ae-Ea/RT. Which of the following options represents the graph of ln k vs 1/T ?
(i)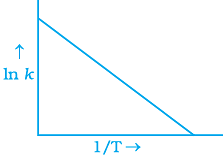
(ii)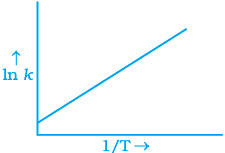
(iii)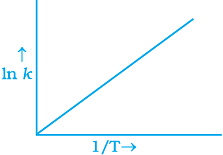
(iv)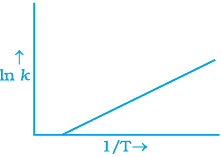
Ans. (i)
Solution.
According to Arrhenius equation,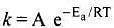
Taking log on both sides in k = In 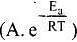
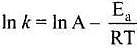
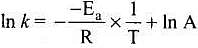
y = mx + c
This equation can be related to equation of straight line.
From the graph, it is very clear that slope of the plot = 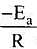 and intercept = in A.
and intercept = in A.
Q.7. Consider the Arrhenius equation given below and mark the correct option.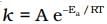
(i) Rate constant increases exponentially with increasing activation energy and decreasing temperature.
(ii) Rate constant decreases exponentially with increasing activation energy and decreasing temperature.
(iii) Rate constant increases exponentially with decreasing activation energy and decreasing temperature.
(iv) Rate constant increases exponentially with decreasing activation energy and increasing temperature.
Ans. (iv)
Solution.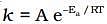 from the equation it is clear that value of rate constant k increases exponentially with decrease in activation energy Ea and increase in temperature.As Ea decreases,
from the equation it is clear that value of rate constant k increases exponentially with decrease in activation energy Ea and increase in temperature.As Ea decreases,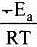 increases and k increases
increases and k increases
As T increases, 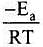 decreases and
decreases and 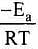 increases and k increases.
increases and k increases.
Q.8. A graph of volume of hydrogen released vs time for the reaction between zinc and dil. HCl is given in Fig. 4.2. On the basis of this mark the correct option.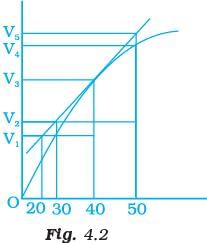
(i) Average rate upto 40s is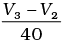
(ii) Average rate upto 40 seconds is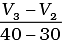
(iii) Average rate upto 40 seconds is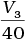
(iv) Average rate upto 40 seconds is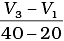
Ans. (iii)
Solution.
Average rate of reaction up to 40 seconds on the basis of the graph is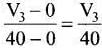
Q.9. Which of the following statements is not correct about order of a reaction.
(i) The order of a reaction can be a fractional number.
(ii) Order of a reaction is experimentally determined quantity.
(iii) The order of a reaction is always equal to the sum of the stoichiometric coefficients of reactants in the balanced chemical equation for a reaction.
(iv) The order of a reaction is the sum of the powers of molar concentration of the reactants in the rate law expression.
Ans. (iii)
Solution.
Out of the given four statements, option (iii) is not correct.
Order of reaction is equal to the sum of powers of concentration of the reactants in rate law expression.
For any chemical reaction
xA + yB → Product
Rate = k[A]x [B]Y
Order = x + y
Order of reaction can be a fraction also. Order of reaction is not always equal to sum of the stoichiometric coefficients of reactants in the balanced chemical equation. For a reaction it may or may not be equal to sum of stoichiometric coefficient of reactants.
Q.10. Consider the graph given in Fig. 4.2. Which of the following options does not show instantaneous rate of reaction at 40th second?
(i)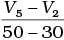
(ii)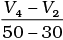
(iii)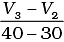
(iv)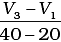
Ans. (ii)
Solution.
Does not show instantaneous rate of reaction at 40th second.
Q.11. Which of the following statements is correct?
(i) The rate of a reaction decreases with passage of time as the concentration of reactants decreases.
(ii) The rate of a reaction is same at any time during the reaction.
(iii) The rate of a reaction is independent of temperature change.
(iv) The rate of a reaction decreases with increase in concentration of reactant(s).
Ans. (i)
Solution.
The rate of a reaction depends upon the concentration of reactants.
Q.12. Which of the following expressions is correct for the rate of reaction given below?
5Br–(aq) + BrO3–(aq) + 6H+(aq) → 3Br2(aq) + 3H2O(l)
(i)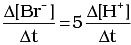
(ii)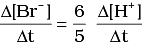
(iii)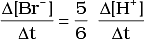
(iv)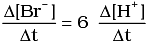
Ans. (iii)
Solution.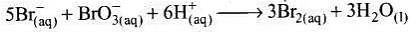
Rate law expression for the above equation can be written as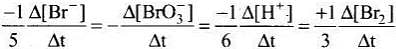
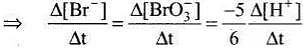
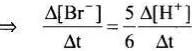
Q.13. Which of the following graphs represents exothermic reaction?
(a)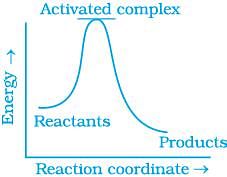
(b)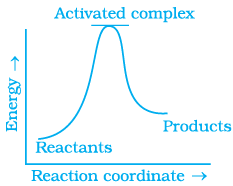
(c)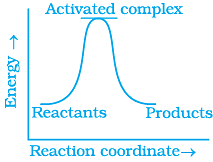
(i) (a) only
(ii) (b) only
(iii) (c) only
(iv) (a) and (b)
Ans. (i)
Solution.
For a exotheramic reaction:
Ea(products) > Ea(reactants)
H(products) < H(reactants)
Q.14. Rate law for the reaction A + 2B → C is found to be
Rate = k [A][B]
Concentration of reactant ‘B’ is doubled, keeping the concentration of ‘A’ constant, the value of rate constant will be______.
(i) The same
(ii) Doubled
(iii) Quadrupled
(iv) Halved
Ans. (i)
Solution.
Rate constant of a reaction does not depend upon concentrations of the reactants.
Q.15. Which of the following statements is incorrect about the collison theory of chemical reaction?
(i) It considers reacting molecules or atoms to be hard spheres and ignores their structural features.
(ii) Number of effective collisions determines the rate of reaction.
(iii) Collision of atoms or molecules possessing sufficient threshold energy results into the product formation.
(iv) Molecules should collide with sufficient threshold energy and proper orientation for the collision to be effective.
Ans. (iii)
Solution.
According the postulates of collision theory there are the following necessary conditions for any reaction to be occur:
(i) Molecule should collide with sufficient threshold energy.
(ii) Their orientation must be proper.
(iii) The collision must be effective
Q.16. A first order reaction is 50% completed in 1.26 × 1014 s. How much time would it take for 100% completion?
(i) 1.26 × 1015 s
(ii) 2.52 × 1014 s
(iii) 2.52 × 1028 s
(iv) infinite
Ans. (iv)
Solution.
Reaction would be 100% complete only after infinite time which cannot be calculated.
Q.17. Compounds ‘A’ and ‘B’ react according to the following chemical equation.
A (g) + 2 B(g) → 2C (g)
Concentration of either ‘A’ or ‘B’ were changed keeping the concentrations of one of the reactants constant and rates were measured as a function of initial concentration. Following results were obtained. Choose the correct option for the rate equations for this reaction.
| Experiment | Initial concentration of [A]/mol L–1 | Initial concentration of [B]/mol L–1 | Initial rate of formation of [C]/mol L–1 s–1 |
| 1. | 0.30 | 0.30 | 0.10 |
| 2. | 0.30 | 0.60 | 0.40 |
| 3. | 0.60 | 0.30 | 0.20 |
(i) Rate = k [A]2 [B]
(ii) Rate = k [A] [B]2
(iii) Rate = k [A] [B]
(iv) Rate = k [A]2 [B]0
Ans. (ii)
Solution.
Rate = K[A]x[B]y
When concentration of B is doubled keeping the concentration of A constant, the rate of formation of C increases by a factor of four. This indicates that the rate of reactions depends upon the square of concentration of B. When concentration of A is doubled, the rate of formation of C also doubles from the initial value. This shows that the rate depends on first power of concentration of A. Hence Rate = K[A]x[B]y
Q.18. Which of the following statement is not correct for the catalyst?
(i) It catalyses the forward and backward reaction to the same extent.
(ii) It alters ∆G of the reaction.
(iii) It is a substance that does not change the equilibrium constant of a reaction.
(iv) It provides an alternate mechanism by reducing activation energy between reactants and products.
Ans. (ii)
Solution.
Characteristics of catalyst
(a) It catalyses the forward and backward reactions to the same extent as it decreases energy of activation hence, increases the rate of both the reactions.
(b) Since reaction quotient is ‘the relation between concentration of reactants and products. Hence, catalyst does not alter Gibbs free energy as it is related reaction quotient. Thus, Gibbs free energy does not change during the reaction when catalyst is added to it.
ΔG = -RT In Q
where Q = reaction quotient
(c) It does not alter equilibrium of reaction as equilibrium constant is also concentration dependent term.
(d) It provides an alternate mechanism by reducing activation energy between reactants and product.
Q.19. The value of rate constant of a pseudo first order reaction ____________.
(i) Depends on the concentration of reactants present in small amount.
(ii) Depends on the concentration of reactants present in excess.
(iii) Is independent of the concentration of reactants.
(iv) Depends only on temperature.
Ans. (ii)
Solution.
The value of rate constant of a pseudo first order reaction depends on the concentration of reactants present in excess.
20. Consider the reaction A ⇌ B. The concentration of both the reactants and the products varies exponentially with time. Which of the following figures correctly describes the change in concentration of reactants and products with time?
(i)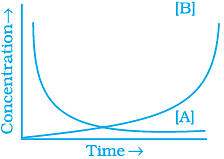
(ii)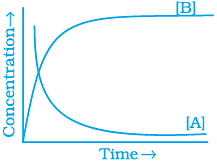
(iii)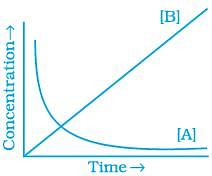
(iv)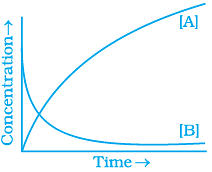
Ans. (ii)
Solution.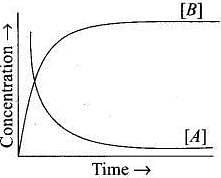
The concentration of reactants decreases with time and concentration of products increases with time.
MULTIPLE CHOICE QUESTIONS
Note : In the following questions two or more options may be correct.
Q.21. Rate law cannot be determined from balanced chemical equation if _______.
(i) Reverse reaction is involved.
(ii) It is an elementary reaction.
(iii) It is a sequence of elementary reactions.
(iv) Any of the reactants is in excess.
Ans. (i, iii, iv)
Solution.
Rate law can be determined from balanced chemical equation if it is an elementary reaction.
Q.22. Which of the following statements are applicable to a balanced chemical equation of an elementary reaction?
(i) Order is same as molecularity.
(ii) Order is less than the molecularity.
(iii) Order is greater than the molecularity.
(iv) Molecularity can never be zero.
Ans. (i, iv)
Solution.
For a balanced chemical equation of an elementary reaction order is same as molecularity and molecularity can never be zero, if molecularity of a reaction is considered to be zero it mean that no reactant is going to transform into product. Consider a chemical reaction.
Q.23. In any unimolecular reaction ______________.
(i) Only one reacting species is involved in the rate determining step.
(ii) The order and the molecularity of slowest step are equal to one.
(iii) The molecularity of the reaction is one and order is zero.
(iv) Both molecularity and order of the reaction are one.
Ans. (i, ii)
Solution.
Since, the reaction is a unimolecular reaction. Hence, in the slowest step i.e., in the rate determining step the only one reacting species is involved. Therefore, order of reaction and molecularity of reaction is equal to one.
Q.24. For a complex reaction ______________.
(i) Order of overall reaction is same as molecularity of the slowest step.
(ii) Order of overall reaction is less than the molecularity of the slowest step.
(iii) Order of overall reaction is greater than molecularity of the slowest step.
(iv) Molecularity of the slowest step is never zero or non integer.
Ans. (i, iv)
Solution.
(i) For a complex reaction, order of overall reaction = molecularity of slowest step As rate of overall reaction depends upon total number of molecules involved in slowest step of the reaction therefore, molecularity of the slowest step is equal to order of overall reaction.
(iv) Since, the completion of any chemical reaction is not possible in the absence of reactants. Hence, slowest step of any chemical reaction must contain at least one reactant. Thus, molecularity of the slowest step is never zero or non-integer.
Q.25. At high pressure the following reaction is zero order.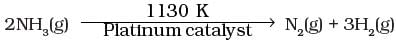
Which of the following options are correct for this reaction?
(i) Rate of reaction = Rate constant
(ii) Rate of the reaction depends on concentration of ammonia.
(iii) Rate of decomposition of ammonia will remain constant until ammonia disappears completely.
(iv) Further increase in pressure will change the rate of reaction.
Ans. (i, iii, iv)
Solution.
Given, chemical reaction is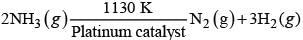
At very high pressure reaction become independent of concentration of ammonia i.e., zero order reaction.
Hence,
Rate = k[pNH3]0
N Rate = k
(a) Rate of reaction = Rate constant
(b) Rate of decomposition of ammonia will remain constant until ammonia disappears completely.
(c) Since, formation of ammonia is a reversible process further increase in pressure will change the rate of reaction. According to Le-Chatelier principle, increase in pressure will favour in backward reaction.
Q.26. During decomposition of an activated complex
(i) Energy is always released
(ii) Energy is always absorbed
(iii) Energy does not change
(iv) Reactants may be formed
Ans. (i, iv)
Solution.
When the reactant molecules collide with each other they lead to formation of an activated complex. When it decomposes to give product, energy is released and stability of product increases. Since, the entire concentration of activated complex do not convert into products while, some activated complex may give reactants also.
Q.27. According to Maxwell Boltzmann distribution of energy, __________.
(i) The fraction of molecules with most probable kinetic energy decreases at higher temperatures.
(ii) The fraction of molecules with most probable kinetic energy increases at higher temperatures.
(iii) Most probable kinetic energy increases at higher temperatures.
(iv) Most probable kinetic energy decreases at higher temperatures.
Ans. (i, iii)
Solution.
Distribution of kinetic energy may be described by plotting a graph of fraction of molecules versus kinetic energy.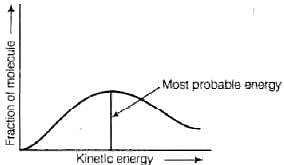 Kinetic energy of maximum fraction of molecules is known as most probable kinetic energy. It is important to note that with increase of temperature, peak shifts forward but downward.
Kinetic energy of maximum fraction of molecules is known as most probable kinetic energy. It is important to note that with increase of temperature, peak shifts forward but downward.
This means that with increase of temperature,
(i) most probable kinetic energy increases.
(ii) the fractions of molecules possessing most probable kinetic energy decreases.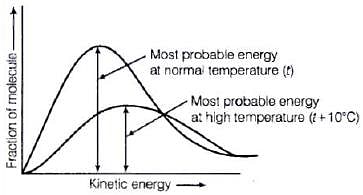
Q.28. In the graph showing Maxwell Boltzman distribution of energy, ___________.
(i) Area under the curve must not change with increase in temperature.
(ii) Area under the curve increases with increase in temperature.
(iii) Area under the curve decreases with increase in temperature.
(iv) With increase in temperature curve broadens and shifts to the right hand side.
Ans. (i, iv)
Solution.
According to Maxwell Boltzmann distribution curve, area under the curve must not change with increase in temperature. But with increase in temperature curve broadens and shift towards right hand side due to decrease in fraction of molecules having most probable kinetic energy.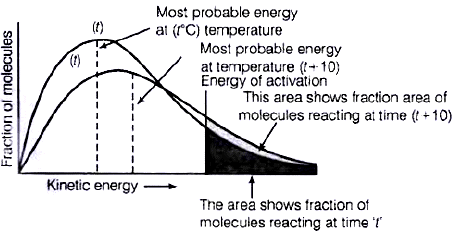
Q.29. Which of the following statements are in accordance with the Arrhenius equation?
(i) Rate of a reaction increases with increase in temperature.
(ii) Rate of a reaction increases with decrease in activation energy.
(iii) Rate constant decreases exponentially with increase in temperature.
(iv) Rate of reaction decreases with decrease in activation energy.
Ans. (i, ii)
Solution.
Arrhenius equation can be written as 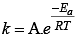
k ∝ e-Ea i.e., rate of reaction increases with decrease in activation energy.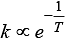
k ∝ eT i.e., rate of reaction increases with increase in temperature.
Q.30. Mark the incorrect statements.
(i) Catalyst provides an alternative pathway to reaction mechanism.
(ii) Catalyst raises the activation energy.
(iii) Catalyst lowers the activation energy.
(iv) Catalyst alters enthalpy change of the reaction.
Ans. (ii, iv)
Solution.
As the catalyst is added to the reaction medium, rate of reaction increases by decreasing activation energy of molecule. Hence, it follows an alternative pathway.
Catalyst does not change the enthalpy change of reaction. Energy of reactant and product remains same in both catalysed and uncatalysed reaction.
Hence, (ii) and (iv) are incorrect statements.
Q.31. Which of the following graphs is correct for a zero order reaction?
(i)
(ii)
(iii)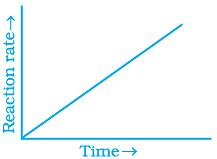
(iv)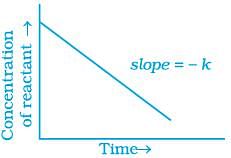
Ans. (i, iv)
Solution.
For a zero order reaction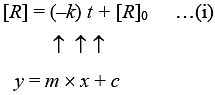
On comparing with equation of straight line
y = [R] concentration
x = t time Slope (m) = –k rate constant
Intercept (c) = [R]0 initial concentration
On rearranging equation (i), we get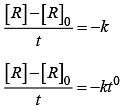
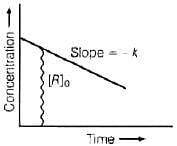
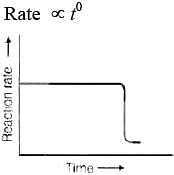
Q.32. Which of the following graphs is correct for a first order reaction?
(i)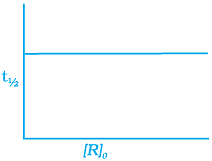
(ii)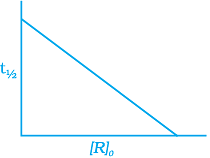
(iii)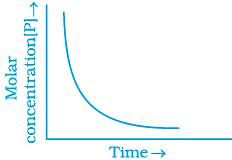
(iv)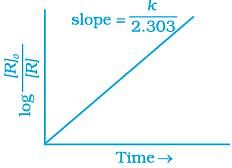
Ans. (i, ii)
Solution.
For the first order reaction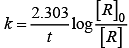
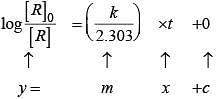
Correct plot of 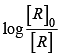 can be represented by (d)
can be represented by (d)
where,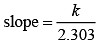
The time taken for any fraction of the reaction to complete is independent of the initial concentration. Let, us consider it for half of the reaction to complete.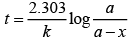
For half-life 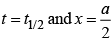
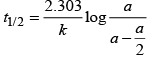
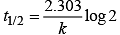
Half-life time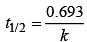
t1/2 is independent of initial concentration. Hence, correct plot of t1/2 and [R]0 can be represented by a.
SHORT ANSWER TYPE QUESTIONS
Q.33. State a condition under which a bimolecular reaction is kinetically first order reaction.
Ans. Bimolecular reaction becomes kinetically first order or pseudo first order reaction when one of the reactant is in excess w.r.t. to other, the rate of reaction depends on one of the reactant only.
e.g., acid catalysed hydrolysis of ethyl acetate.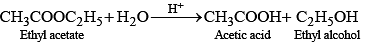
This reaction is bimolecular but is found to be of first order as experimentally it is observed that rate of reaction depends upon the concentration of ethyl acetate not on water as it is present in excess.
Q.34. Write the rate equation for the reaction 2A + B → C if the order of the reaction is zero.
Ans. For reaction 2A + B → C if the rate of reaction is zero then it can be represented as
Rate = k [A]0 [B] = k
i.e., rate of reaction is independent of concentration of A and B.
Q.35. How can you determine the rate law of the following reaction?
2NO (g) + O2 (g) → 2NO2 (g)
Ans. We can determine the rate of this reaction as a function of initial concentrations either by keeping the concentration of one of the reactants constant and changing the concentration of the other reactant or by changing the concentration of both the reactants.
e.g., for the given reaction,
(i) Keeping [O2] constant, if the concentration of NO is doubled, rate is found to become four times. This shows that,
Rate ∝ [NO]2
(ii) Keeping [NO] constant, if the concentration of [O2] is doubled, rate is also found to become double. This shows that,
Rate ∝ [O2]2
Hence, overall rate law will be
Rate = k [NO]2[O2]
Rate law expression= 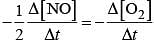
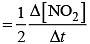
Q.36. For which type of reactions, order and molecularity have the same value?
Ans. If the reaction is elementary reaction then order and molecularity have same value because elementary reaction proceeds in a single step.
Q.37. In a reaction if the concentration of reactant A is tripled, the rate of reaction becomes twenty seven times. What is the order of the reaction?
Ans. Rate of any elementary reaction can be represented as
r = k[A]n
After changing concentration to its triple value A = 3A r becomes 27r
27r = k[3A]n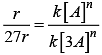
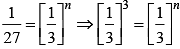
Hence, n = 3
Thus, order of reaction is three.
Q.38. Derive an expression to calculate time required for completion of zero order reaction.
Ans. For zero order reaction [R] = [R]0 – kt
For completion on of the reaction [R] = 0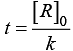
Q.39. For a reaction A + B → Products, the rate law is — Rate = k[A][B]3/2. Can the reaction be an elementary reaction? Explain.
Ans. During an elementary reaction, the number or atoms or ions colliding to react is referred to as molecularity. Had this been an elementary reaction, the order of reaction with respect to B would have been 1, but in the given rate law it is 3/2. This indicates that the reaction is not an elementary reaction. Hence, this reaction must be a complex reaction.
Q.40. For a certain reaction large fraction of molecules has energy more than the threshold energy, yet the rate of reaction is very slow. Why?
Ans. According to collision theory apart from the energy considerations, the colliding molecules should also have proper orientation for effective collision.
This condition might not be getting fulfilled in the reaction as it shows the number of reactants taking part in a reaction, which can never be zero.
Q.41. For a zero order reaction will the molecularity be equal to zero? Explain.
Ans. No, the molecularity can never be zero or a fractional number as it shows the number of reactants taking part in a reaction which can never be zero.
Q.42. For a general reaction A → B, plot of concentration of A vs time is given in Fig. 4.3. Answer the following question on the basis of this graph.
(i) What is the order of the reaction?
(ii) What is the slope of the curve?
(iii) What are the units of rate constant?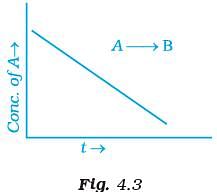 Ans. (i) For A → B the given graph shows a zero order reaction. Mathematically represented as
Ans. (i) For A → B the given graph shows a zero order reaction. Mathematically represented as
[R] = –kt + [R]0
which is equation of straight line. Hence, reaction is a zero order.
(ii) Slope = – k
(iii) Unit of zero order reaction is mole L–1 s–1.
Q.43. The reaction between H2(g) and O2(g) is highly feasible yet allowing the gases to stand at room temperature in the same vessel does not lead to the formation of water. Explain.
Ans. Because activation energy of the reaction is very high at room temperature but at high temperature H — H and O — O bond break and colliding particles cross the energy barrier. This is why reaction between H2(g) and O2(g) does not lead to formation of water at room temperature while keeping in the same vessel.
Q.44. Why does the rate of a reaction increase with rise in temperature?
Ans. At higher temperatures, larger fraction of colliding particles can cross the energy barrier (i.e., the activation energy) which leads to faster rate.
Q.45. Oxygen is available in plenty in air yet fuels do not burn by themselves at room temperature. Explain.
Ans. For combustion reactions, activation energy of fuels is very high at room temperature. So, fuels do not burn by themselves at room temperature.
Q.46. Why is the probability of reaction with molecularity higher than three very rare?
Ans. According to collision theory, we know that to complete any chemical reaction there must be effective collision between reactant particles and they must have minimum sufficient energy. The probability of more than three molecules colliding simultaneously is very small. Hence, possibility of molecularity being three is very low.
Q.47. Why does the rate of any reaction generally decreases during the course of the reaction?
Ans. The rate of a reaction depends on the concentration of the reactants. As the reaction proceeds in forward direction, concentration of reactant decreases and that of products increases. So, the rate of reaction generally decreases during the course of reaction.
Q.48. Thermodynamic feasibility of the reaction alone cannot decide the rate of the reaction. Explain with the help of one example.
Ans. Thermodynamically the conversion of diamond to graphite is highly feasible but this reaction is very slow because its activation energy is high.
Hence, thermodynamic feasibility of the reaction alone cannot decide the rate of reaction.
Q.49. Why in the redox titration of KMnO4 vs oxalic acid, we heat oxalic acid solution before starting the titration?
Ans. As we know with increase in temperature rate of reaction increases. Hence, we heat oxalic acid solution before starting of titration to increase the rate of decolourisation.
Q.50. Why can’t molecularity of any reaction be equal to zero?
Ans. Molecularity of the reaction is the number of molecules taking part in an elementary step. For this we require at least a single molecule leading to the value of minimum molecularity of one. Hence, molecularity of any reaction can never be equal to zero.
Q.51. Why molecularity is applicable only for elementary reactions and order is applicable for elementary as well as complex reactions?
Ans. A complex reaction occurs through a number of steps i.e., elementary reactions. Number of molecuies involved in each elementary reaction may be different, i.e., the molecularity of each step may be different. Therefore, it is meaningless to talk of molecularity of the overall complex reaction.
On the other hand, order of complex reaction depends upon the molecularity of the slowest step. Hence, it is not meaningless to talk of the order of a complex reaction.
Q.52. Why can we not determine the order of a reaction by taking into consideration the balanced chemical equation?
Ans. Balanced chemical equation often leads to incorrect order or rate law. e.g., the following reaction seems to be a tenth order reaction:
KClO3 + 6FeSO4 + 3H2SO4 → KCI + 3H2O + 3H2(SO4)3
This is actually a second order reaction. Actually the reaction is complex and occurs in several steps. The order of such reaction is determined by the slowest step in the reaction mechanism.
Order is determined experimentally and is confined to the dependence of observed rate of reaction on the concentration of reactants.
MATCHING TYPE
Note : In the following questions match the items of Column I with appropriate item given in Column II.
Q.53. Match the graph given in Column I with the order of reaction given in Column II.
More than one item in Column I may link to the same item of Column II.
| Column I | Column II |
(i) 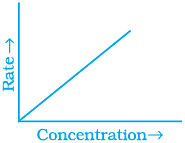 | |
(ii) 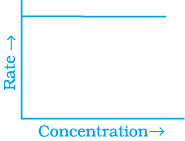 | (a) Ist order |
(iii) 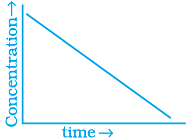 | (b) Zero order |
(iv) 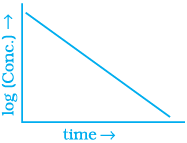 |
Ans. (i) → (a) (ii) → (b) (iii) → (b) (iv) → (a)
Solution.
For zero order reaction rate equation may be written as
[R] = –kt + [R0] ...(i)
Which denotes a straight line equation similar to y = m x + c
On transforming (i) 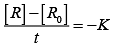
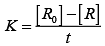
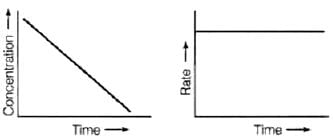 K = Rate
K = Rate
Rate = k.[t]°
For a first order reaction 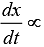 [concentration]
[concentration]
∴ Graph between rate and concentration may be drawn as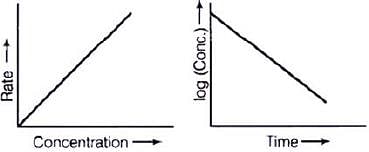
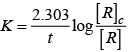
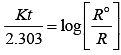
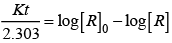
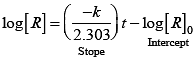
Q.54. Match the statements given in Column I and Column II
| Column I | Column II |
| (i) Catalyst alters the rate of reaction | (a) Cannot be fraction or zero |
| (ii) Molecularity | (b) Proper orientation is not there always |
| (iii) Second half life of first order reaction | (c) By lowering the activation energy |
| (iv) e–Ea/RT | (d) Is same as the first |
| (v) Energetically favourable reactions are sometimes slow | (e) Total probability is one |
| (vi) Area under the Maxwell Boltzman curve is constant | (f) Refers to the fraction of molecules with energy equal to or greater than activation energy |
Ans. (i) → (c) (ii) → (a) (iii) → (d) (iv) → (f) (v) → (2) (vi) → (e)
Solution.
1. Catalyst alters the rate of reaction by lowering activation energy
2. Molecularity can't be fraction or zero. If molecularity is zero, then reaction is not possible.
3. Second half-life of first order reaction is same as first because half-life time is temperature independent.
4. e-Ea/RT refers to the fraction of molecules with kinetic energy equal to or greater than activation energy.
5. Energetically favourable reactions are sometimes slow due to improper orientation of molecule cause some ineffective collision of molecules.
6. Area under the Maxwell, Boltzmann curve is constant because total probability of molecule taking part in a chemical reaction is equal to one.
Q.55. Match the items of Column I and Column II.
| Column I | Column II |
| (i) Diamond | (a) Short interval of time |
| (ii) Instantaneous rate | (b) Ordinarily rate of conversion is imperceptible |
| (iii) Average rate | (c) Long duration of time |
Ans. (i) → (b) (ii) → (a) (iii) → (c)
Solution.
1. Diamond can't be converted into graphite under ordinary condition.
2. Instantaneous rate of reaction completes at very short span of time.
3. Average rate of reaction occurs to a long duration of time.
Q.56. Match the items of Column I and Column II.
| Column I | Column II |
| (i) Mathematical expression for rate of reaction | (a) Rate constant |
| (ii) Rate of reaction for zero order reaction is equal to | (b) Rate law |
| (iii) Units of rate constant for zero order reaction is same as that of | (c) Order of slowest step |
| (iv) Order of a complex reaction is determined by | (d) Rate of a reaction |
Ans. (i) → (b) (ii) → (a) (iii) → (d) (iv) → (c)
Solution.
1. Mathematical expression for rate of reaction is known as rate law.
2. Rate of reaction for zero order reaction is equal to rate constant
r = k[A]°
r = k
3. Unit of rate of reaction is same as that of rate of reaction.
4. Order of complex reaction is determined by rate of a reaction, which is slowest.
ASSERTION AND REASON TYPE
Note: In the following questions a statement of assertion followed by a statement of reason is given.
Choose the correct answer out of the following choices.
(i) Both assertion and reason are correct and the reason is correct explanation of assertion.
(ii) Both assertion and reason are correct but reason does not explain assertion.
(iii) Assertion is correct but reason is incorrect.
(iv) Both assertion and reason are incorrect.
(v) Assertion is incorrect but reason is correct.
Q.57. Assertion : Order of the reaction can be zero or fractional.
Reason : We cannot determine order from balanced chemical equation.
(i) Both assertion and reason are correct and the reason is correct explanation of assertion.
(ii) Both assertion and reason are correct but reason does not explain assertion.
(iii) Assertion is correct but reason is incorrect.
(iv) Both assertion and reason are incorrect.
(v) Assertion is incorrect but reason is correct.
Ans. (ii)
Solution.
Assertion is correct as order can be zero or fraction but it can be determined experimentally.
Q.58. Assertion : Order and molecularity are same.
Reason : Order is determined experimentally and molecularity is the sum of the stoichiometric coefficient of rate determining elementary step.
(i) Both assertion and reason are correct and the reason is correct explanation of assertion.
(ii) Both assertion and reason are correct but reason does not explain assertion.
(iii) Assertion is correct but reason is incorrect.
(iv) Both assertion and reason are incorrect.
(v) Assertion is incorrect but reason is correct.
Ans. (v)
Solution.
Order and molecularity may or may not be same as order of reaction is sum of power of reactant which can be determined experimentally. But molecularity is sum of stoichiometric coefficient of rate determining elementary step.
Q.59. Assertion : The enthalpy of reaction remains constant in the presence of a catalyst.
Reason : A catalyst participating in the reaction, forms different activated complex and lowers down the activation energy but the difference in energy of reactant and product remains the same.
(i) Both assertion and reason are correct and the reason is correct explanation of assertion.
(ii) Both assertion and reason are correct but reason does not explain assertion.
(iii) Assertion is correct but reason is incorrect.
(iv) Both assertion and reason are incorrect.
(v) Assertion is incorrect but reason is correct.
Ans. (i)
Solution.
Enthalpy of reaction i.e., difference of total enthalpy of reactants and product remains constant in the presence of a catalyst. As a catalyst participating in the reaction forms different activated complex and lowers down the activation energy but the difference in energy of reactant and product remains same.
Q.60. Assertion : All collision of reactant molecules lead to product formation.
Reason : Only those collisions in which molecules have correct orientation and sufficient kinetic energy lead to compound formation.
(i) Both assertion and reason are correct and the reason is correct explanation of assertion.
(ii) Both assertion and reason are correct but reason does not explain assertion.
(iii) Assertion is correct but reason is incorrect.
(iv) Both assertion and reason are incorrect.
(v) Assertion is incorrect but reason is correct.
Ans. (v)
Solution.
Every collision among reactant molecules does not lead to the formation of product. Only effective collision brings out the formation of product.
Q.61. Assertion : Rate constants determined from Arrhenius equation are fairly accurate for simple as well as complex molecules.
Reason : Reactant molecules undergo chemical change irrespective of their orientation during collision.
(i) Both assertion and reason are correct and the reason is correct explanation of assertion.
(ii) Both assertion and reason are correct but reason does not explain assertion.
(iii) Assertion is correct but reason is incorrect.
(iv) Both assertion and reason are incorrect.
(v) Assertion is incorrect but reason is correct.
Ans. (iii)
Solution.
Rate constant determined from Arrhenius equation are fairly accurate for simple and complex molecules because only those molecules which have proper orientation during collision (i.e., effective collision) and sufficient kinetic energy lead the chemical change.
LONG ANSWER TYPE QUESTIONS
Q.62. All energetically effective collisions do not result in a chemical change. Explain with the help of an example.
Ans. Only effective collision leads to the formation of products. It means that collisions in which molecules collide with sufficient kinetic energy (called threshold energy = activation energy + energy possessed by reacting species) and proper orientation lead to a chemical change because it facilitates the breaking of old bonds between (reactant) molecules and formation of the new ones i.e.,in products. e.g., formation of methanol from bromomethane depends upon the orientation of the reactant molecules.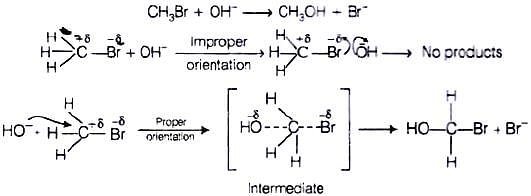
The proper orientation of reactant molecules leads to bond formation whereas improper orientation makes them simply back and no products are formed.
To account for effective collisions, another factor P (probability or steric factor) is introduced. It takes into account the fact that in a collision, molecules must be properly oriented i.e.,
Rate, K = PZABe-Ea/RT
Q.63. What happens to most probable kinetic energy and the energy of activation with increase in temperature?
Ans. Kinetic energy is directly proportional to the absolute temperature and the number of molecules possessing higher energies increases with increase in temperature, i.e., most probable kinetic energy increases with increase in temperature.
Energy of activation is related to temperature by the following Arrhenius equation
K = Ae- Ea/RT
Thus, it also shows an increase with rise in temperature.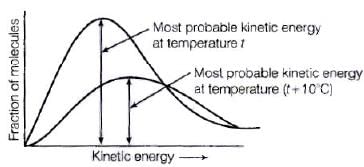
Q.64. Describe how does the enthalpy of reaction remain unchanged when a catalyst is used in the reaction.
Ans. A catalyst is a substance which increases the speed of a reaction without itself undergoing any chemical change.
According to "intermediate complex formation theory" reactants first combine with the catalyst to form an intermediate complex which is short-lived and decomposes to form the products and regenerating the catalyst.
The intermediate formed has much lower potential energy than the intermediate complex formed between the reactants in the absence of the catalyst.
Thus, the presence of catalyst lowers the potential energy barrier and the reaction follows a new alternate pathway which requires less activation energy.
We know that, lower the activation energy, faster is the reaction because more reactant molecules can cross the energy barrier and change into products.
Enthalpy, ΔH is a state function. Enthalpy of reaction, i.e., difference in energy between reactcints and product is constant, which is clear from potential energy diagram.
Potential energy diagram of catalysed reaction is given as: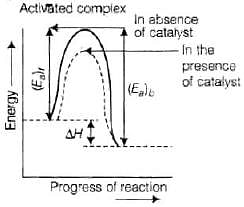
Q.65. Explain the difference between instantaneous rate of a reaction and average rate of a reaction.
Ans.
| S.No. | Instantaneous rate of reaction | Average rate of reaction |
| (i) | It occurs within a short span of time. | It occurs during a long interval of time. |
| (ii) | It can't be calculated for multistep reaction. | It can be calculated for multistep reaction. |
| (iii) | It can be calculated for elementary reaction. | It can be calculated for elementary reaction. |
Q.66. With the help of an example explain what is meant by pseudo first order reaction.
Ans. A reaction in which one reactant is present in large amount and its concentration does not get altered during the course of the reaction, behaves as first order reaction. Such reaction is called pseudo first order reaction, e.g., (i) hydrolysis of ethyl acetate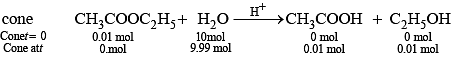
Rate of reaction = K [CH3COOC2H5]
where, k = k' [H2O]
e.g., (ii) inversion of cane sugar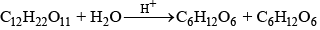
Rate of reacSon = K [C12H22O11]
where k = k'[H2O]
|
108 videos|286 docs|123 tests
|
FAQs on NCERT Exemplar: Chemical Kinetics - Chemistry Class 12 - NEET
| 1. What is chemical kinetics? |  |
| 2. What are the factors that affect the rate of a chemical reaction? |  |
| 3. How can the rate of a chemical reaction be determined experimentally? |  |
| 4. What is the role of a catalyst in a chemical reaction? |  |
| 5. How can the order of a chemical reaction be determined? |  |

|
Explore Courses for NEET exam
|

|












