NCERT Exemplar: Classification of Elements & Periodicity in Properties - 1 | Chemistry Class 11 - NEET PDF Download
Multiple Choice Questions (Type - I)
Q.1. Consider the isoelectronic species, Na+, Mg2+, F– and O2–. The correct order of increasing length of their radii is ______.
(i) F- < O2– < Mg2+ < Na+
(ii) Mg2+ < Na+ < F– < O2–
(iii) O2– < F– < Na+ < Mg2+
(iv) O2– < F– < Mg2+ < Na+
Ans. (ii)
Solution.
All of them are isoelectronic species. They have the same number of electrons (10). Their radii would be different because of their different nuclear charges. The cation with the greater positive charge will have a smaller radius because of the greater attraction of the electrons to the nucleus. Anion with the greater negative charge will have a larger radius. In this case, the net repulsion of the electrons will outweigh the nuclear charge and the ion will expand in size.
Q.2. Which of the following is not an Actinoid?
(i) Curium (Z = 96)
(ii) Californium (Z = 98)
(iii) Uranium (Z = 92)
(iv) Terbium (Z = 65)
Ans. (iv)
Solution.
Actinoids are elements with Z= 90-103. They are characterised by the outer electronic configuration (n-2) f-14 (n-1) dns2. The last electron added to each element is filled in f-orbital. Hence Terbium (Z = 65) is not an actinoid. It is a lanthanoid.
Q.3. The order of screening effect of electrons of s, p, d and f orbitals of a given shell of an atom on its outer shell electrons is:
(i) s > p > d > f
(ii) f > d > p > s
(iii) p < d < s > f
(iv) f > p > s > d
Ans. (i)
Solution.
The effective nuclear charge experienced by a valence electron in an atom will be less than the actual charge on the nucleus because of ‘‘shielding” or “screening” of the valence electron from the nucleus by the intervening core electrons.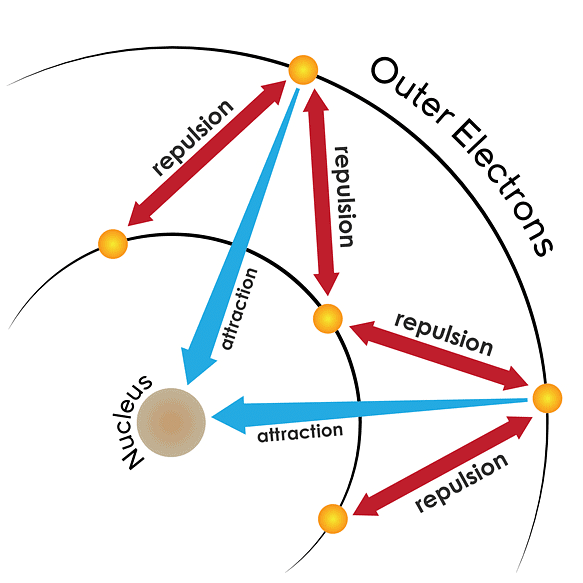 Screening EffectExample: the 2s electron in lithium is shielded from the nucleus by the inner core of 1s electrons.
Screening EffectExample: the 2s electron in lithium is shielded from the nucleus by the inner core of 1s electrons.
Q.4. The first ionisation enthalpies of Na, Mg, Al and Si are in the order:
(i) Na < Mg > Al < Si
(ii) Na > Mg > Al > Si
iii) Na < Mg < Al < Si
(iv) Na > Mg > Al < Si
Ans. (i)
Solution.
- The electronic configurations of Na and Mg are:
Na (11): [Ne] 3s1 and Mg (12): [Ne] 3s2
In both the atoms, the electron is to be removed from 3s-orbital but nuclear charge in Na is less than Mg. Thus, ionisation energy of Na is less than Mg (Na < Mg). - The electronic configurations of Mg and Al are:
Mg: [Ne] 3s2; Al: [Ne] 3s2 3pl
In Mg, the electron is to be removed from 3s-orbital while in Al, it is to be removed from 3p-orbital. Since it is easier to remove an electron from 3p-orbital in comparison to 3s orbital, the ionization enthalpy of Mg is higher than Al (Mg > Al). - The electronic configurations of Al and Si are:
Al (13): [Ne] 3s2 3p1 Si (14): [Ne] 3s2 3p2
In both the atoms, the electron is to be removed from 3p-orbital but nuclear charge in Si is more than Al. Thus, ionisation enthalpy of Al is less than Si.
Q.5. The electronic configuration of gadolinium (Atomic number 64) is
(i) [Xe] 4f3 5d5 6s2
(ii) [Xe] 4f7 5d2 6s1
(iii) [Xe] 4f7 5d1 6s2
(iv) [Xe] 4f8 5d6 6s2
Ans. (iii)
The electronic configuration of La (Z = 57) is [Xe] 5dl 6s2. Therefore, further addition of electrons occurs in the lower energy 4f-orbital till it is exactly half-filled at Eu (Z = 63) Thus, the electronic configuration of Eu is [Xe] 4f7 6s2. Thereafter, addition of next electron does not occur in the more stable exactly half-filled 4f7 shell but occurs in the little higher energy 5d-orbital. Thus, the electronic configuration of Gd (Z = 64) is [Xe] 4f7 5dl 6s2.
Q.6. The statement that is not correct for periodic classification of elements is:
(i) The properties of elements are periodic function of their atomic numbers. (ii) Non metallic elements are less in number than metallic elements.
(iii) For transition elements, the 3d-orbitals are filled with electrons after 3p-orbitals and before 4s-orbitals.
(iv) The first ionisation enthalpies of elements generally increase with increase in atomic number as we go along a period.
Ans. (iii)
Solution.
For transition elements, the 3d-orbitals are filled with electrons after 3p and 4s-orbitals and before 4p-orbitals. The order of filling the orbitals is:
1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s …
Q.7. Among halogens, the correct order of amount of energy released in electron gain (electron gain enthalpy) is:
(i) F > Cl > Br > I
(ii) F < Cl < Br < I
(iii) F < Cl > Br > I
(iv) F < Cl < Br < I
Ans. (iii)
- Chlorine has higher electron gain enthalpy than fluorine. This is due to small size of fluorine atom, i.e., the electron density is high which resists the addition of an electron (F < Cl).
- In general, electron gain enthalpy decreases as atomic size increases. Thus, electron gain enthalpy follows the order:
Cl > Br > I
Q.8. The period number in the long form of the periodic table is equal to
(i) magnetic quantum number of any element of the period.
(ii) atomic number of any element of the period.
(iii) maximum Principal quantum number of any element of the period.
(iv) maximum Azimuthal quantum number of any element of the period.
Ans. (iii)
Solution.
Since each period starts with the filling of electrons in a new principal quantum number, therefore, the period number in the long form of the periodic table refers to the maximum principal quantum number of any element in the period.
Period number = maximum n of any element
(where, n = principal quantum number).
Q.9. The elements in which electrons are progressively filled in 4f-orbital are called
(i) actinoids
(ii) transition elements
(iii) lanthanoids
(iv) halogens
Ans. (iii)
Solution.
The sixth period (n = 6) contains 32 elements and successive electrons enter 6s, 4f, 5d and 6p orbitals, in the order of filling up of the 4f orbitals begins with cerium (Z = 58) and ends at lutetium (Z = 71) to give the 4f-inner transition series which is called the lanthanoid series.
Q.10. Which of the following is the correct order of size of the given species:
(i) I > I– > I+
(ii) I+ > I– > I
(iii) I > I+ > I–
(iv) I– > I > I+
Ans. (iv)
Solution.
A cation is smaller than its parent atom because it has fewer electrons while its nuclear charge remains the same. The size of an anion will be larger than that of the parent atom because the addition of one or more electrons would result in increased repulsion among the electrons and a decrease in effective nuclear charge.
Q.11. The formation of the oxide ion, O2– (g), from oxygen atom requires first an exothermic and then an endothermic step as shown below:
O (g) + e– → O– (g) ; ∆ HΘ = - 141 kJ mol-1
O– (g) + e– → O2– (g); ∆ HΘ = + 780 kJ mol-1
Thus process of formation of O2– in gas phase is unfavourable even though O2– is isoelectronic with neon. It is due to the fact that,
(i) oxygen is more electronegative.
(ii) addition of electron in oxygen results in larger size of the ion.
(iii) electron repulsion outweighs the stability gained by achieving noble gas configuration.
(iv) O– ion has comparatively smaller size than oxygen atom.
Ans. (iii)
Solution.
When an electron is added to O atom to form O- ion, energy is released. Thus, the first electron gain enthalpy of O is negative. On the other hand, when an electron is added to O- ion to form O2-ion, energy has to be given out in order to overcome the strong electronic repulsions. Thus the second electron gain enthalpy of O is positive.
Q.12. Comprehension given below is followed by some multiple choice questions. Each question has one correct option. Choose the correct option.
"In the modern periodic table, elements are arranged in order of increasing atomic numbers which is related to the electronic configuration. Depending upon the type of orbitals receiving the last electron, the elements in the periodic table have been divided into four blocks, viz, s, p, d and f. The modern periodic table consists of 7 periods and 18 groups. Each period begins with the filling of a new energy shell. In accordance with the Arfbau principle, the seven periods (1 to 7) have 2, 8, 8, 18, 18, 32 and 32 elements respectively. The seventh period is still incomplete. To avoid the periodic table being too long, the two series of f-block elements, called Lanthanoids and actinoids are placed at the bottom of the main body of the periodic table."
(a) The element with atomic number 57 belongs to
(i) s-block
(ii) p-block
(iii) d-block
(iv) f-block
Ans. (iii)
Solution.
Lanthanoids which are characterised by the filling of 4f-orbitals, are the elements following lanthanum from 58Ce to 71Lu. Actinoids characterised by filling of 5f-orbitals, are the elements following actinium from 90Th to 103Lr. Characteristic outer electronic configuration is (n - 2)f14 (n - 1)d0-1ns2.
(b) The last element of the p-block in 6th period is represented by the outermost electronic configuration.
(i) 7s2 7p6
(ii) 5f14 6d10 7s2 7p0
(iii) 4f14 5d10 6s2 6p6
(iv) 4f14 5d10 6s2 6p4
Ans. (iii)
Solution.
The sixth period (n = 6) contains 32 elements and successive electrons enter 6s, 4f, 5d and 6p orbitals. The last element in the p-block in 6th period is 86Rn.
(c) Which of the elements whose atomic numbers are given below, cannot be accommodated in the present set up of the long form of the periodic table?
(i) 107
(ii) 118
(iii) 126
(iv) 102
Ans. (iii)
Solution.
The present set up of the long form of the periodic table has seven periods and four blocks. So, the maximum number of elements according to Aufbau principle which can be accommodated are 118.
(d) The electronic configuration of the element which is just above the element with atomic number 43 in the same group is ______.
(i) 1s2 2s2 2p6 3s2 3p6 3d5 4s2
(ii) 1s2 2s2 2p6 3s2 3p6 3d5 4s2 4p6
(iii) 1s2 2s2 2p6 3s2 3p6 3d6 4s2
(iv) 1s2 2s2 2p6 3s2 3p6 3d7 4s2
Ans. (i)
Solution.
The atomic number of the element which lies just above the element with atomic number 43 is 25 (Mn). Its electronic configuration is 1s2 2s2 2p6 3s2 3p6 3d5 4s2.
(e) The elements with atomic numbers 35, 53 and 85 are all ______.
(i) noble gases
(ii) halogens
(iii) heavy metals
(iv) light metals
Ans. (ii)
Solution.
The elements with atomic numbers 35, 53 and 85 lie in a group before noble gases i.e., halogens (group 17). Thus, the elements with atomic numbers 35, 53 and 85 are halogens.
Q.13. Electronic configurations of four elements A, B, C and D are given below:
(A) 1s2 2s2 2p6
(B) 1s2 2s2 2p4
(C) 1s2 2s2 2p6 3s1
(D) 1s2 2s2 2p5
Which of the following is the correct order of increasing tendency to gain electron :
(i) A < C < B < D
(ii) A < B < C < D
(iii) D < B < C < A
(iv) D < A < B < C
Ans. (i)
A – 1s2 2s2 2p6 – Noble gas configuration
B -1s2 2s2 2p4 – 2 electrons short of stable configuration
C – 1s2 2s2 2p6 3.?1 – Requires one electron to complete 5-orbital
D -1s2 2s2 2p5 – Requires one electron to attain noble gas configuration
- Noble gases have no tendency to gain electrons since all their orbitals are completely filled. Thus, element A has the least electron gain enthalpy.
- Since element D has one electron less and element B has two electrons less than the corresponding noble gas configuration, hence, element D has the highest electron gain enthalpy followed by element B.
- Since, element C has one electron in the 5-orbital and hence needs one more electron to complete it, therefore, electron gain enthalpy of C is less than that of element B. Combining all the facts given above, the electron gain enthalpies of the four elements increase in the order A < C < B < D.
Multiple Choice Questions (Type - II)
In the following questions two or more options may be correct.
Q.14. Which of the following elements can show covalency greater than 4?
(i) Be
(ii) P
(iii) S
(iv) B
Ans. (ii) and (iii)
Solution.
P and S have d-orbitals in their valence shell and therefore, can accommodate more than 8. electrons in their respective valence shells. Hence they show covalency more than 4.
Q.15. Those elements impart colour to the flame on heating in it, the atoms of which require low energy for the ionisation (i.e., absorb energy in the visible region of spectrum). The elements of which of the following groups will impart colour to the flame?
(i) 2
(ii) 13
(iii) 1
(iv) 17
Ans. (i) and (iii)
Solution.
The elements of group 1 (alkali metals) and group 2 (alkaline earth metals) have low ionization enthalpies. Therefore, they impart colour to flame.
Q.16. Which of the following sequences contain atomic numbers of only representative elements?
(i) 3, 33, 53, 87
(ii) 2, 10, 22, 36
(iii) 7, 17, 25, 37, 48
(iv) 9, 35, 51, 88
Ans. (i) and (iv)
Solution.
Elements of 5 and p-block elements are called representative elements. Elements of f-block (Z=21 – 30; 39 – 48; 57 and 72 – 80; 89 and 104 – 112) are called transition elements while those of f-block (with Z = 58-71 and Z = 90 – 103) are called inner transition elements.
(i) 3 – Group 1, 33 – group 15, 53 – group 17 and 87 – group 1.
(ii) 9 – Group 17, 35 – Group 17, 51 – Group 15, 88 – Group 2.
Q.17. Which of the following elements will gain one electron more readily in comparison to other elements of their group?
(i) S (g)
(ii) Na (g)
(iii) O (g)
(iv) Cl (g)
Ans. (i) and (iv)
Solution.
For many elements energy is released when an electron is added to the atom and the electron gain enthalpy is negative. For example, group 17 elements (the halogens) have very high negative electron gain enthalpies because they can attain stable noble gas electronic configurations by picking up an electron.
Q.18. Which of the following statements are correct?
(i) Helium has the highest first ionisation enthalpy in the periodic table.
(ii) Chlorine has less negative electron gain enthalpy than fluorine.
(iii) Mercury and bromine are liquids at room temperature.
(iv) In any period, atomic radius of alkali metal is the highest.
Ans. (i), (iii) and (iv)
Chlorine has more negative electron gain enthalpy than fluorine due to bigger size and lesser electronic repulsion.
Q.19. Which of the following sets contain only isoelectronic ions?
(i) Zn2+, Ca2+, Ga3+, Al3+
(ii) K+, Ca2+, Sc3+, Cl –
(iii) P3–, S2–, Cl–, K+
(iv) Ti4+, Ar, Cr3+, V5+
Ans. (ii) and (iii)
Solution.
(i) Zn2+ (30 – 2 = 28), Ca2+ (20 – 2 = 18), Ga3+ (31-3= 28), Al3+ (13 – 3 = 10) are not isoelectronic.
(ii) K+ (19 – 1 = 18), Ca2+ (20 – 2 = 18), Sc3+ (21 – 3 = 18), Cl– (17 + 1 = 18) are isoelectronic.
(iii) P3- (15 + 3 = 18), S2- (16 + 2 = 18), Cl– (17 + 1 = 18), K+ (19 – 1 = 18) are isoelectronic.
(iv) Ti4+ (22 – 4 = 18), Ar (18), Cr3+ (24 – 3 = 21), V5+ (23 – 5 = 18) are not isoelectronic.
Q.20. In which of the following options order of arrangement does not agree with the variation of property indicated against it?
(i) Al3+ < Mg2+ < Na+ < F– (increasing ionic size)
(ii) B < C < N < O (increasing first ionisation enthalpy)
(iii) I < Br < Cl < F (increasing electron gain enthalpy)
(iv) Li < Na < K < Rb (increasing metallic radius)
Ans. (ii) and (iii)
The ionisation enthalpy of N is higher than that of O due to greater stability of half-filled electronic configuration. Hence (ii) is incorrect.
Again in option (iii), the electron gain enthalpy of F is lower than that of Cl due to small size of F. Hence (iii) is wrong.
Q.21. Which of the following have no unit?
(i) Electronegativity
(ii) Electron gain enthalpy
(iii) Ionisation enthalpy
(iv) Metallic character
Ans. (i) and (iv)
Solution.
A qualitative measure of the ability of an atom in a chemical compound to attract shared electrons to itself is called electronegativity. Unlike ionization enthalpy and electron gain enthalpy, it is not a measureable quantity.
Q.22. Ionic radii vary in
(i) inverse proportion to the effective nuclear charge.
(ii) inverse proportion to the square of effective nuclear charge.
(iii) direct proportion to the screening effect.
(iv) direct proportion to the square of screening effect.
Ans. (i) and (iii)
Solution.
The cation with the greater positive charge will have a smaller radius because of the greater attraction of the electrons to the nucleus. Anion with the greater negative charge will have the larger radius. In this case, the net repulsion of the electrons will outweigh the nuclear charge and the ion will expand in size so the ionic radii vary in inverse proportion to the effective nuclear charge and direct proportion to the screening effect.
Q.23. An element belongs to 3rd period and group-13 of the periodic table. Which of the following properties will be shown by the element?
(i) Good conductor of electricity
(ii) Liquid, metallic
(iii) Solid, metallic
(iv) Solid, non metallic
Ans. (i, iii) The element belonging to 3rd period and 13th group is aluminium which is a metal. Hence, it is solid, metallic and good conductor of electricity.
|
129 videos|244 docs|88 tests
|
FAQs on NCERT Exemplar: Classification of Elements & Periodicity in Properties - 1 - Chemistry Class 11 - NEET
| 1. What is the periodic table of elements? |  |
| 2. How many elements are there in the periodic table? |  |
| 3. What is the significance of periods and groups in the periodic table? |  |
| 4. What are the main characteristics of metals, nonmetals, and metalloids in the periodic table? |  |
| 5. How does the atomic radius change across a period and down a group in the periodic table? |  |

|
Explore Courses for NEET exam
|

|


















