NCERT Solutions Class 11 Chemistry Chapter 16 - Chemistry in Everyday Life
Question 16.1: Why do we need to classify drugs in different ways?
Answer
The classification of drugs and the reasons for classification are as follows:
(i) On the basis of pharmacological effect:
This classification provides doctors with the whole range of drugs available for the treatment of a particular type of problem. Hence, such a classification is very useful to doctors.
(ii) On the basis of drug action:
This classification is based on the action of a drug on a particular biochemical process. Thus, this classification is important.
(iii) On the basis of chemical structure:
This classification provides the range of drugs sharing common structural features and often having similar pharmacological activity.
(iv) On the basis of molecular targets:
This classification provides medicinal chemists with the drugs having the same mechanism of action on targets. Hence, it is the most useful to medicinal chemists.
Question 16.2: Explain the term target molecules or drug targets as used in medicinal chemistry.
Answer
In medicinal chemistry, drug targets refer to the key molecules involved in certain metabolic pathways that result in specific diseases. Carbohydrates, proteins, lipids, and nucleic acids are examples of drug targets.
Drugs are chemical agents designed to inhibit these target molecules by binding with the active sites of the key molecules.
Question 16.3: Name the macromolecules that are chosen as drug targets.
Answer
The macromolecules chosen as drug targets are carbohydrates, lipids, proteins, and nucleic acids.
Question 16.4: Why should not medicines be taken without consulting doctors?
Answer
A medicine can bind to more than one receptor site. Thus, a medicine may be toxic for some receptor sites. Further, in most cases, medicines cause harmful effects when taken in higher doses than recommended. As a result, medicines may be poisonous in such cases. Hence, medicines should not be taken without consulting doctors.
Question 16.5: Define the term chemotherapy.
Answer
The use of chemicals for therapeutic effect is called chemotherapy. For example the use of chemicals in the diagnosis, prevention, and treatment of diseases
Question 16.6: Which forces are involved in holding the drugs to the active site of enzymes?
Answer
Either of the following forces can be involved in holding drugs to the active sites of enzymes.
(i) Ionic bonding
(ii) Hydrogen bonding
(iii) Dipole-dipole interaction
(iv) van der Waals force
Question 16.7: While antacids and antiallergic drugs interfere with the function of histamines, why do these not interfere with the function of each other?
Answer
Specific drugs affect particular receptors. Antacids and anti-allergic drugs work on different receptors. This is the reason why antacids and anti-allergic drugs do not interfere with each other’s functions but interfere with the functions of histamines.
Question 16.8: Low level of noradrenaline is the cause of depression. What types of drugs are needed to cure this problem? Name two drugs.
Answer
Anti-depressant drugs are needed to counteract the effect of depression. These drugs inhibit enzymes catalysing the degradation of the neurotransmitter, noradrenaline. As a result, the important neurotransmitter is slowly metabolised and then it can activate its receptor for longer periods of time.
Two anti-depressant drugs are:
(i) Iproniazid
(ii) Phenelzine
Question 16.9: What is meant by the term ‘broad-spectrum antibiotics? Explain.
Answer
Antibiotics that are effective against a wide range of gram-positive and gram-negative bacteria are known as broad-spectrum antibiotics. Chloramphenicol is a broad-spectrum antibiotic.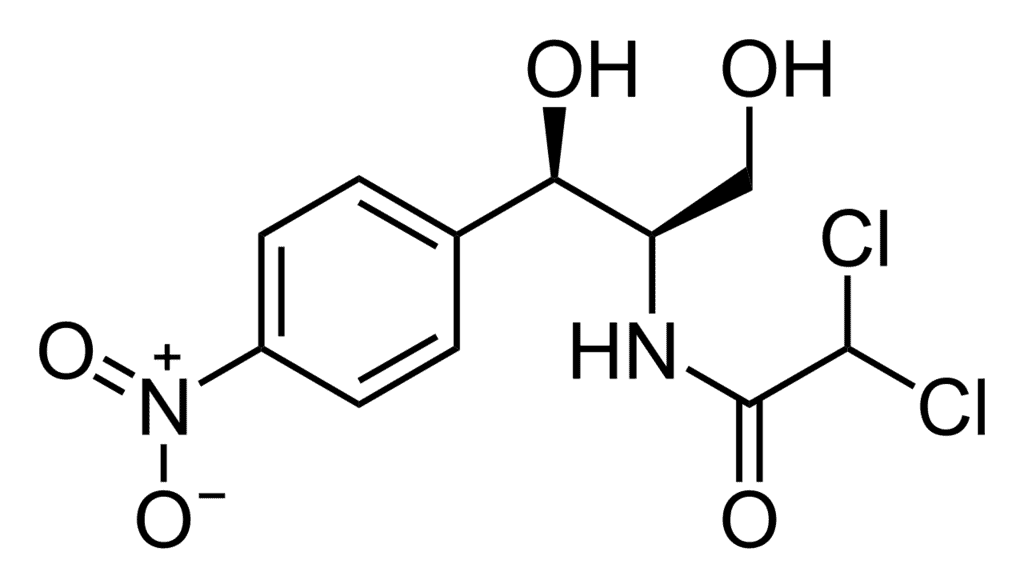 Chloramphenicol StructureIt can be used for the treatment of typhoid, dysentery, acute fever, pneumonia, meningitis, and certain forms of urinary infections. Two other broad-spectrum antibiotics are vancomycin and ofloxacin. Ampicillin and amoxicillin −synthetically modified from penicillin − are also broad-spectrum antibiotics.
Chloramphenicol StructureIt can be used for the treatment of typhoid, dysentery, acute fever, pneumonia, meningitis, and certain forms of urinary infections. Two other broad-spectrum antibiotics are vancomycin and ofloxacin. Ampicillin and amoxicillin −synthetically modified from penicillin − are also broad-spectrum antibiotics.
Question 16.10: How do antiseptics differ from disinfectants? Give one example of each.
Answer
Antiseptics and disinfectants are effective against micro-organisms. However, antiseptics are applied to the living tissues such as wounds, cuts, ulcers, and diseased skin surfaces, while disinfectants are applied to inanimate objects such as floors, drainage system,
instruments, etc. Disinfectants are harmful to live tissues.
Iodine is an example of a strong antiseptic. A tincture of iodine (2 − 3 per cent of solution of iodine in the alcohol-water mixture) is applied to wounds. 1 per cent solution of phenol is used as a disinfectant.
Question 16.11: Why are cimetidine and ranitidine better antacids than sodium hydrogen carbonate or magnesium or aluminium hydroxide?
Answer
Antacids such as sodium hydrogen carbonate, magnesium hydroxide, and aluminium hydroxide work by neutralising the excess hydrochloric acid present in the stomach. However, the root cause for the release of excess acid remains untreated.
Cimetidine and ranitidine are better antacids as they control the root cause of acidity.
These drugs prevent the interaction of histamine with the receptors present in the stomach walls. Consequently, there is a decrease in the amount of acid released by the stomach. This is why cimetidine and ranitidine are better antacids than sodium hydrogen carbonate, magnesium hydroxide, and aluminium hydroxide.
Question 16.12: Name a substance that can be used as an antiseptic as well as a disinfectant.
Answer
Phenol can be used as an antiseptic as well as a disinfectant. 0.2 per cent solution of phenol is used as an antiseptic, while 1 per cent of its solution is used as a disinfectant.
Question 16.13: What are the main constituents of Dettol?
Answer
The main constituents of Dettol are chloroxylenol and α-terpineol.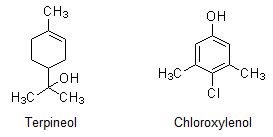
Question 16.14:
What is the tincture of iodine? What is its use?
Answer
The tincture of iodine is a 2 − 3 per cent solution of iodine in an alcohol-water mixture. It is applied to wounds as an antiseptic.
Question 16.15: What are food preservatives?
Answer
Food preservatives are chemicals that prevent food from spoilage due to microbial growth. Table salt, sugar, vegetable oil, sodium benzoate (C6H3COONa), and salts of propanoic acid are some examples of food preservatives.
Question 16.16: Why is the use of aspartame limited to cold foods and drinks?
Answer
Aspartame becomes unstable at cooking temperature. This is the reason why its use is limited to cold foods and drinks.
Question 16.17: What are artificial sweetening agents? Give two examples.
Answer
Artificial sweetening agents are chemicals that sweeten food. However, unlike natural sweeteners, they do not add calories to our body. They do not harm the human body.
Some artificial sweeteners are aspartame, saccharin, sucrolose, and alitame.
Question 16.18: Name a sweetening agent used in the preparation of sweets for a diabetic patient.
Answer
Artificial sweetening agents such as saccharin, alitame, and aspartame can be used in preparing sweets for diabetic patients.
Question 16.19: What problem arises in using alitame as an artificial sweetener?
Answer
Alitame is a high potency sweetener. It is difficult to control the sweetness of food while using alitame as an artificial sweetener.
Question 16.20: How are synthetic detergents better than soap?
Answer
Soaps work in soft water. However, they are not effective in hard water. In contrast, synthetic detergents work both in soft water and hard water.
Therefore, synthetic detergents are better than soaps.
Question 16.21: Explain the following terms with suitable examples
(i) Cationic detergents
(ii) Anionic detergents and
(iii) Non-ionic detergents
Answer
(i) Cationic detergent
Cationic detergents are quaternary ammonium salts of acetates, chlorides, or bromides.
These are called cationic detergents because the cationic part of these detergents contains a long hydrocarbon chain and a positive charge on the N atom.
For example cetyltrimethylammonium bromide
(ii) Anionic detergents
Anionic detergents are of two types:
1.Sodium alkyl sulphates: These detergents are sodium salts of long-chain alcohols. They are prepared by first treating these alcohols with concentrated sulphuric acid and then with sodium hydroxide. Examples of these detergents include sodium lauryl sulphate (C11H23CH2OSO3 −Na+) and sodium stearyl sulphate (C17H35CH2OSO3 −Na+).
2.Sodium alkyl benzene sulphonates: These detergents are sodium salts of long-chain alkyl benzene sulphonic acids. They are prepared by Friedel-Crafts alkylation of benzene with long-chain alkyl halides or alkenes. The obtained product is first treated with concentrated sulphuric acid and then with sodium hydroxide. Sodium 4-(1-dodecyl) benzenesulfonate (SDS) is an example of anionic detergents.
(iii) Non-ionic detergents
The molecules of these detergents do not contain any ions. These detergents are esters of alcohols having high molecular mass. They are obtained by reacting polyethene glycol and stearic acid.
Question 16.22: What are biodegradable and non-biodegradable detergents? Give one example of each.
Answer
Detergents that can be degraded by bacteria are called biodegradable detergents. Such
detergents have straight hydrocarbon chains. For example, sodium lauryl sulphate Detergents that cannot be degraded by bacteria are called non-biodegradable detergents. Such detergents have highly-branched hydrocarbon chains. For example: sodium -4- (1, 3, 5, 7- tetramethyl octyl) benzene sulphonate
Question 16.23: Why do soaps not work in hard water?
Answer
Soaps are sodium or potassium salts of long-chain fatty acids. Hard water contains calcium and magnesium ions. When soaps are dissolved in hard water, these ions displace sodium or potassium from their salts and form insoluble calcium or magnesium salts of fatty acids. These insoluble salts separate as scum. This is the reason why soaps do not work in hard water.
Question 16.24: Can you use soaps and synthetic detergents to check the hardness of water?
Answer
Soaps get precipitated in hard water, but not in soft water. Therefore, soaps can be used for checking the hardness of the water.
However, synthetic detergents do not get precipitated either in hard water or in soft water. Therefore, synthetic detergents cannot be used for checking the hardness of the water.
Question 16.25: Explain the cleansing action of soaps.
Answer
Soap molecules form micelles around an oil droplet (dirt) in such a way that the hydrophobic parts of the stearate ions attach themselves to the oil droplet and the hydrophilic parts project outside the oil droplet. Due to the polar nature of the hydrophilic parts, the stearate ions (along with the dirt) are pulled into the water, thereby removing the dirt from the cloth.
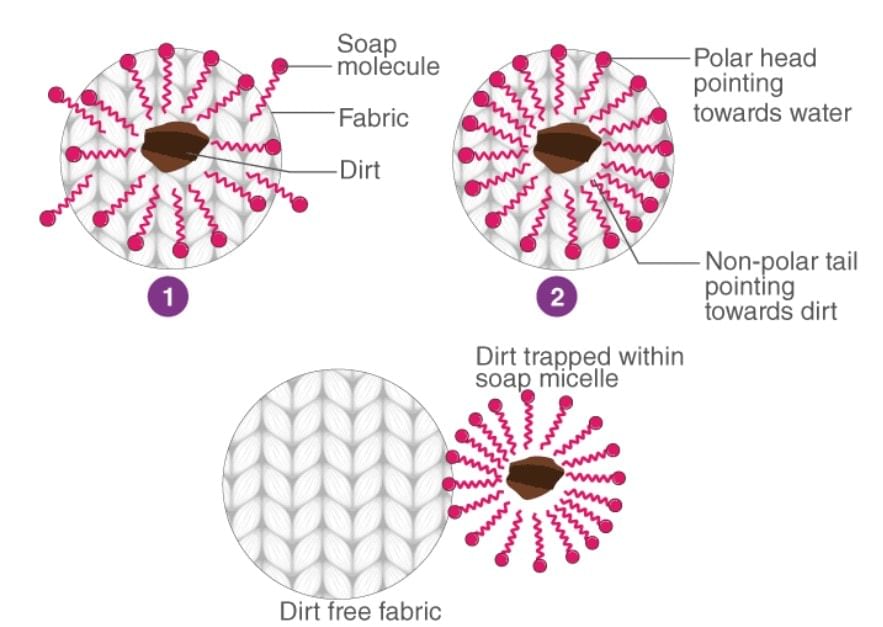
Question 16.26: If the water contains dissolved calcium hydrogen carbonate, out of soaps and synthetic detergents which one will you use for cleaning clothes?
Answer
Synthetic detergents are preferred for cleaning clothes. When soaps are dissolved in water containing calcium ions, these ions form insoluble salts that are of no further use.
However, when synthetic detergents are dissolved in water containing calcium ions, these ions form soluble salts that act as cleansing agents.
|
366 videos|824 docs|301 tests
|
FAQs on NCERT Solutions Class 11 Chemistry Chapter 16 - Chemistry in Everyday Life
| 1. What is the importance of chemistry in everyday life? |  |
| 2. How are chemicals used in the pharmaceutical industry? |  |
| 3. What are the risks associated with the excessive use of chemicals in everyday life? |  |
| 4. How do additives in food and beverages affect our health? |  |
| 5. How does chemistry contribute to environmental pollution? |  |

















