NEET Previous Year Questions (2014-2024): Electrochemistry | Chemistry Class 12 PDF Download
2024
Q1: Match List I with List II.
Choose the correct answer from the options given below :
(a) A-II, B-IV, C-I, D-III
(b) A-III, B-IV, C-I, D-II
(c) A-II, B-III, C-I, D-IV
(d) A-III, B-IV, C-II, D-I (NEET 2024)
Ans: (a)
To answer this question, we need to calculate the number of Faraday's required for each of the reactions in List I.
Reaction A: Conversion of H2O to O2
The reaction for electrolysis of water to produce oxygen can be written as:
Each mole of O2 requires the transfer of 4 moles of electrons. Therefore, to produce 1 mole of O2, you need 4 Faraday's of charge. However, since the reaction shown in the table suggests the production of only 1 mole, it would require 2 Faraday's. This is because we are dealing with a half reaction in which only 2 moles of electrons are required for every mole of O2 produced from 1 mole of H2O.
Reaction B: Conversion of MnO4- to Mn2+
The reduction reaction for permanganate to manganese ion is:
This reaction indicates that 5 moles of electrons are involved in the reduction of 1 mole of MnO4- to Mn2+. Therefore, 5 Faraday's are required.
Reaction C: Production of 1.5 moles of Ca from molten CaCl2
The electrolytic production of calcium from its chloride can be illustrated as follows:
Each mole of Ca produced requires 2 Faraday's of electrons considering the transfer of 2 moles of electrons. For 1.5 moles of Ca, the total Faraday's required would be 3 Faraday's, because, 1.5 * 2 = 3F.
Reaction D: Oxidation of FeO to Fe2O3
The reaction can be considered in terms of iron oxidation states:
Each mole of O2 requires 2 Faraday's of charge. If the products include 2 moles of Fe2O3 and 1 mole of O2, thus, only 2 Faraday's will be required for 1 mole of FeO to react (considering FeO as just a part of the full reaction).
Correct Matching:
A-II, B-IV, C-I, D-III
The mappings are A-II (2F for H2O to O2), B-IV (5F for MnO4- to Mn2+), C-I (3F for 1.5 mol of Ca from CaCl2), and D-III (2F for FeO to Fe2O3).
Therefore, the correct answer is Option A:
A-II, B-IV, C-I, D-III.
Q2: Mass in grams of copper deposited by passing 9.6487 A current through a voltmeter containing copper sulphate solution for 100 seconds is (Given : Molar mass of Cu : 63 g mol−1,1 F = 96487C)
(a) 3.15 g
(b) 0.315 g
(c) 31.5 g
(d) 0.0315 g (NEET 2024)
Ans: (b)
The mass of copper deposited when passing an electric current through a copper sulphate solution can be calculated using Faraday's laws of electrolysis. The first law states that the amount of a substance deposited or liberated at an electrode during electrolysis is proportional to the amount of electricity (charge) passed through the electrolyte.
To find the mass of copper deposited, we use the formula:
Where:
m is the mass of the substance deposited (in grams),
M is the molar mass of the substance (in g/mol),
I is the current (in amperes, A),
t is the time electricity is passed through the solution (in seconds),
n is the number of moles of electrons required to deposit or dissolve 1 mole of the substance (valence number, which is 2 for copper in copper sulphate solution as copper ions are Cu2+),F is the Faraday constant, approximately 96487C/mol of electrons.
Given:
Substitute these values into the formula:
Hence, the mass of copper deposited is approximately 0.315 g, making Option B the correct answer.
2023
Q.1. The conductivity of centimolar solution of KCl at 25°C is 0.0210 ohm–1 cm–1 and the resistance of the cell containing the solution at 25°C is 60 ohm. The value of cell constant is (NEET 2023)(a) 1.34 cm–1
(b) 3.28 cm–1
(c) 1.26 cm–1
(d) 3.34 cm–1
Ans: (c)

Q.2. Given below are two statements: one is labelled as Assertion A and the other is labelled as Reason R
Assertion A : In equation △rG = –nFEcell’ value of △rG depends on n.
Reasons R: Ecell is an intensive property and △rG is an extensive property.
In the light of the above statements, choose the correct answer from the options given below
(a) Both A and R are true and R is the correct explanation of A
(b) Both A and R are true and R is NOT the correct explanation of A
(c) A is true but R is false
(d) A is false but R is true
Ans: (b)
2022
Q1: Two half cell reactions are given below. (NEET 2022 Phase 2)

The standard EMF of a cell with feasible redox reaction will be :
(a) −3.47 V
(b) +7.09 V
(c) +0.15 V
(d) +3.47 V
Ans: (d)
Since E0OP of Al is more than Co2+, so at anode Al will oxidise and at cathode Co3+ will reduce.

Q2: Standard electrode potential for the cell with cell reaction (NEET 2022 Phase 2)
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
is 1.1 V. Calculate the standard Gibbs energy change for the cell reaction. (Given F = 96487 C mol−1)
(a) −200.27 J mol−1
(b) −200.27 kJ mol−1
(c) −212.27 kJ mol−1
(d) −212.27 J mol−1
Ans: (c)
Q3: At 298 K, the standard electrode potentials of Cu2+ / Cu, Zn2+/Zn, Fe2+/Fe and Ag+/Ag are 0.34 V, – 0.76 V, – 0.44 V and 0.80 V, respectively. On the basis of standard electrode potential, predict which of the following reaction can not occur?
(a) FeSO4(aq) + Zn(s) → ZnSO4(aq) + Fe(s)
(b) 2CuSO4(aq) + 2Ag(s) → 2Cu(s) + Ag2SO4(aq)
(c) CuSO4(aq) + Zn(s) → ZnSO4(aq) + Cu(s)
(d) CuSO4(aq) + Fe(s) → FeSO4(aq) + Cu(s) (NEET 2022 Phase 1)
Ans: (b)
(a)
= 0.44 ( 0.76)
= + 0.32 V ( will occurs)
(b)
= 0.34 (0.80)
= -0.46 V ( will not occurs)
(c) 
= 0.34 ( 0.76 )
= + 1.10 V ( will occurs)
(d) 
= 0.34 ( 0.44 )
= + 0.78 V ( will occurs)
Q4: Given below are half-cell reactions: (NEET 2022 Phase 1)
Will the permanganate ion, liberate O2 from water in the presence of an acid?
liberate O2 from water in the presence of an acid?
(a) Yes, because E°cell = + 2.733 V
(b) No, because E°cell = – 2.733 V
(c) Yes, because E°cell = + 0.287 V
(d) No, because E°cell = – 0.287 V
Ans: (c)
= 1.51 - 1.223
= +0.287 V
Since E°cell is positive, O2 will be released.
Q5: Find the emf of the cell in which the following reaction takes place at 298 K
Ni(s) + 2Ag+ (0.001 M) → Ni2+(0.001 M) + 2Ag(s)
(Given that  at 298 K)
at 298 K)
(a) 0.9615 V
(b) 1.05 V
(c) 1.0385 V
(d) 1.385 V (NEET 2022 Phase 1)
Ans: (a)

= 1.05 – 0.0885
= 0.9615 V
2021
Q1: The molar conductance of NaCl, HCI, and CH3COONa at infinite dilution are 126.45, 426.16, and 91.0 S cm mol-1 respectively. The molar conductance of CH3COOH at infinite dilution is. Choose the right option for your answer. (NEET 2021)
(a) 698.28 ohm-1 cm2 mol-1
(b) 540.48 ohm-1 cm2 mol-1
(c) 201.28 ohm-1 cm2 mol-1
(d) 390.71 ohm-1 cm2 mol-1
Ans: (d)
According to Kohlrausch law of independent migration of ions.
Q2: The molar conductivity of 0.007 M acetic acid is 20 S cm2 mol-1. What is the dissociation constant of acetic acid? Choose the correct option. (2021)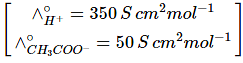
(a) 1.75 × 10–5 mol L–1
(b) 2.50 × 10–5 mol L–1
(c) 1.75 × 10–4 mol L–1
(d) 2.50 × 10–4 mol L–1
Ans: (a)




2020
Q1: On electrolysis of dil. sulphuric acid using platinum (Pt) electrode, the product obtained at the anode will be: (NEET 2020)
(a) H2S gas
(b) SO2 gas
(c) Hydrogen gas
(d) Oxygen gas
Ans: (d)
During the electrolysis of dil. sulphuric acid using Pt electrodes following reaction will take place.
At cathode :
At anode :
At the anode oxygen gas will be released.
Q2: The number of Faradays (F) required to produce 20g of calcium from molten CaCl2 (Atomic mass of Ca = 40 g mol-1) is:
(a) 3
(b) 4
(c) 1
(d) 2 (NEET 2020)
Ans: (c)
1 equivalent of any substance is deposited by 1F of charge.
We have 20g calcium.
No. of equivalent = given mass / equivalent mass
Ca2+ + 2e-
v.f. = 2
According to Faraday's 1st law
Charge passed in Faradey = g. equivalent of product
=
So, 1 F of charge is required.
2019
Q1: For a cell involving one electron E°cell = 0.59 V at 298 K, the equilibrium constant for the cell reaction is :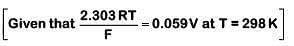 (NEET 2019)
(NEET 2019)
(a) 1.0 × 102
(b) 1.0 × 105
(c) 1.0 × 1010
(d) 1.0 × 1030
Ans: (c)
We know,
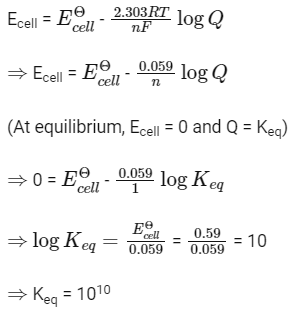
Q2: For the cell reaction
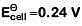 at 298 K. The standard Gibbs energy
at 298 K. The standard Gibbs energy 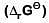 of the cell reaction is :[Given that Faraday constant F = 96500 C mol–1] (NEET 2019)
of the cell reaction is :[Given that Faraday constant F = 96500 C mol–1] (NEET 2019)
(a) – 46.32 kJ mol–1
(b) – 23.16 kJ mol–1
(c) 46.32 kJ mol–1
(d) 23.16 kJ mol–1
Ans: (a)
Here, n = 2
ΔGo = -nFEo
= – 2 × 96500 × 0.24
= – 46320 J mol–1
= – 46.32 KJ mol–1
2018
Q1: Consider the change in the oxidation state of Bromine corresponding to different emf values as shown in the diagram below: Then the species undergoing disproportionation is:- (NEET 2018)
Then the species undergoing disproportionation is:- (NEET 2018)
(a) BrO3-
(b) BrO4-
(c) Br2
(d) HBrO
Ans: (d)
Calculate Eocell corresponding to each compound undergoing disproportionation reaction. The reaction for which Eocell comes out + ve is spontaneous.
HBrO → Br2, EoHBrO/Br2 = 1.595 V
HBrO → BrO3–, EoBrO3-/HBrO = 1.595 V
Eocell for the disproportionation of HBrO,
Eocell = EoHBrO/Br2 - EoBrO3-/HBrO
= 1.595 - 1.5
= 0.095 V = + ve
So, Eocell > 0
So, ΔGo < 0 (Spontaneous)
2017
Q1: In the electrochemical cell :  the emf of this Daniell cell is E1. When the concentration of ZnSO4 is changed to 1.0 M and that of CuSO4 changed to 0.01 M, the emf changes to E2. From the followings, which one is the relationship between E1 and E2? (Given, RT/F = 0.059) (NEET 2017)
the emf of this Daniell cell is E1. When the concentration of ZnSO4 is changed to 1.0 M and that of CuSO4 changed to 0.01 M, the emf changes to E2. From the followings, which one is the relationship between E1 and E2? (Given, RT/F = 0.059) (NEET 2017)
(a) E1 < E2
(b) E1 > E2
(c) E2 = 0 ≠ E1
(d) E1 = E2
Ans: (b)

2016
Q1: The molar conductivity of a 0.5 mol/dm3 solution of AgNO3 with electrolytic conductivity of 5.76
(a) 2.88 S cm2/mol
(b) 11.52 S cm2/mol
(c) 0.086 S cm2/mol
(d) 28.8 S cm2/mol (NEET 2016 Phase 2)
Ans: (b)

Q2: If the Eocell for a given reaction has a negative value, which of the following gives the correct relationships for the values of ΔGo and Keq ?
(a) ΔGo > 0; Keq < 1
(b) ΔGo > 0; Keq > 1
(c) ΔGo < 0; Keq > 1
(d) ΔGo < 0; Keq < 1 (NEET 2016 Phase 2)
Ans: (a)
We know that

Q3: During the electrolysis of molten sodium chloride, the time required to produce 0.10 mol of chlorine gas using a current of 3 amperes is (NEET 2016 Phase 2)
(a) 55 minutes
(b) 110 minutes
(c) 220 minutes
(d) 330 minutes
Ans: (b)
At cathode : 2Na+ + 2e–
At anode : 2Cl–
----------------------------------------------
Net reaction: 2Na+ + 2Cl–
From Faraday’s first law of electrolysis,

= 6433.33 sec
= 107.22 min ≃ 110 min
Q4: Zinc can be coated on iron to produce galvanized iron but the reverse is not possible. It is because
(a) zinc is lighter than iron
(b) zinc has lower melting point than iron
(c) zinc has lower negative electrode potential than iron
(d) zinc has higher negative electrode potential than iron (NEET 2016 Phase 2)
Ans: (d)
The reduction potential values are
E°Zn2+/Zn = – 0.76 V
E°Fe2+/Fe = – 0.44 V
Thus, due to higher negative electro potential value of zinc than iron, iron cannot be coated on zinc.
Q5: The number of electrons delivered at the cathode during electrolysis by a current of 1 ampere in 60 seconds is (charge on electron = 1.60 × 10−19C)
(a) 6 × 1023
(b) 6 × 1020
(c) 3.75 × 1020
(d) 7.48 × 1023 (NEET 2016 Phase 2)
Ans: (c)
Q = I × t
Q = 1 × 60 = 60 C
Now, 1.60 × 10–19 C ≡ 1 electron

Q6: The pressure of H2 required to make the potential of H2-electrode zero in pure water at 298 K is : (NEET 2016 Phase 1)
(a) 10-4 atm
(b) 10-14 atm
(c) 10-12 atm
(d) 10-10 atm
Ans: (b)
pH = 7 for water.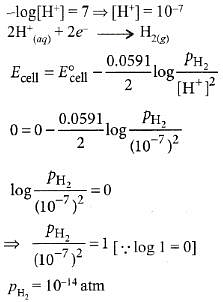
2015
Q1: Aqueous solution of which of the following compounds is the best conductor of electric current ?
(a) Hydrochloric acid, HCl
(b) Ammonia, NH3
(c) Fructose, C6H12O6
(d) Acetic acid, C2H4O2 (NEET / AIPMT 2015)
Ans: (a)
HCl completely dissociates to give H+ and Cl- ions, hence act as very good electrolyte. While others are non- electrolytes.
Q2: A device that converts the energy of combustion of fuels like hydrogen and methane, directly into electrical energy is known as (NEET / AIPMT 2015 Cancelled Paper)
(a) Ni-Cd cell
(b) Fuel Cell
(c) Electrolytic Cell
(d) Dynamo
Ans: (b)
A device that converts energy of combustion of fuels, directly into electrical energy is known as fuel cell.
2014
Q1: When 0.1 mol 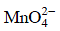 is oxidised the quantity of electricity required to completely oxidise
is oxidised the quantity of electricity required to completely oxidise is :
is :
(a) 9650 C
(b) 96.50 C
(c) 96500 C
(d) 2 × 96500 C (NEET / AIPMT 2014)
Ans: (a)
The oxidation reaction is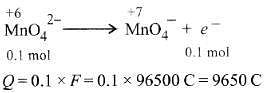
Q2: The weight of silver (at. wt. = 108) displaced by a quantity of electricity that displaces 5600 mL of O2 at STP will be :
(a) 54.0 g
(b) 108.0 g
(c) 5.4 g
(d) 10.8 g (NEET / AIPMT 2014)
Ans: (b)
According to Faraday's second law,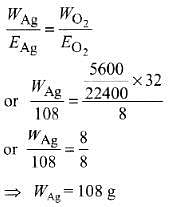
|
108 videos|286 docs|123 tests
|
FAQs on NEET Previous Year Questions (2014-2024): Electrochemistry - Chemistry Class 12
| 1. What is electrochemistry? |  |
| 2. What are some applications of electrochemistry? |  |
| 3. What is an electrochemical cell? |  |
| 4. What is the difference between galvanic cell and electrolytic cell? |  |
| 5. What is the significance of standard electrode potential in electrochemistry? |  |

|
Explore Courses for NEET exam
|

|


















