NEET Previous Year Questions (2014-2024): Coordination Compounds | Chemistry Class 12 PDF Download
2024
Q1: Match List I with List II. Choose the correct answer from the options given below:
Choose the correct answer from the options given below:(a) A-II, B-III, C-IV, D-I
(b) A-I, B-III, C-IV, D-II
(c) A-I, B-IV, C-III, D-II
(d) A-II, B-IV, C-III, D-I (NEET 2024)
Ans: (a)

Q2: Given below are two statements :
Statement I : [Co(NH3)6]3+ is a homoleptic complex whereas [Co(NH3)4Cl2]+ is a heteroleptic complex.
Statement II : Complex [Co(NH3)6]3+ has only one kind of ligands but [Co(NH3)4Cl2]+ has more than one kind of ligands.
In the light of the above statements, choose the correct answer from the options given below.
(a) Both Statement I and Statement II are true
(b) Both Statement I and Statement II are false
(c) Statement I is true but Statement II is false
(d) Statement I is false but Statement II is true (NEET 2024)
Ans: (a)
To determine the correctness of these statements and choose the correct option, we need to understand the concepts of homoleptic and heteroleptic complexes:
Homoleptic Complex: A complex in which a central atom is surrounded by only one kind of donor atom or identical ligands is known as a homoleptic complex. An example would be [Co(NH3)6]3+, where all the ligands are ammonia (NH3).
Heteroleptic Complex: A complex in which a central atom is surrounded by more than one kind of donor atom or different ligands is known as a heteroleptic complex, such as [Co(NH3)4Cl]+ which contains two different types of ligands, ammonia (NH3) and chloride (Cl−).
Statement I: [Co(NH3)6]3+ is a homoleptic complex whereas [Co(NH3)4Cl2]+ is a heteroleptic complex.
This statement is accurate because [Co(NH3)6]3+ indeed is a homoleptic complex as it consists only of ammonia ligands. Conversely,
[Co(NH3)4Cl2]+ is a heteroleptic complex, containing both ammonia and chloride ligands.
Statement II: Complex [Co(NH3)6]3+ has only one kind of ligands but [Co(NH3)4Cl2]+ has more than one kind of ligands.
This statement is also true. As explained, in [Co(NH3)6]3+, there is only one type of ligand (ammonia), and in [Co(NH3)4Cl2]+, there are two different types of ligands (ammonia and chloride), which is consistent with the definition provided.
Conclusion: Both Statement I and Statement II are true. Therefore, the correct choice is:
Option A: Both Statement I and Statement II are true
2023
Q1: Homoleptic complex from the following complexes is (NEET 2023)
(a) Potassium trioxalatoaluminate (III)
(b) Diamminechloridonitrito-N-platinum (II)
(c) Pentaamminecarbonatocobalt (III) chloride
(d) Triamminetriaquachromium (III) chloride
Ans: (a)
- Complexes in which a metal is bound to only one kind of donor groups are called as homoleptic complexes
- Potassium trioxalatoaluminate (III)
K3[Al(ox)3]
It is a homoleptic complex
Q2: Which complex compound is most stable? (NEET 2023)
(a) [Co(NH3)4(H2O)Br]NO3)2
(b) [Co(NH3)3(NO3)3]
(c) [CoCl2(en)2]NO3
(d) [Co(NH3)6] 2(SO4)3
Ans: (c)
Chelating ligands in general form more stable complexes than their monodentate analogs
∴ The most stable complex is
[CoCl2(en)2]NO3
2022
Q1: Match List-I with List-II : (NEET 2022 Phase 2)

Choose the correct answer from the options given below :
(a) (a)-(iv), (b)-(iii), (c)-(ii), (d)-(i)
(b) (a)-(iii), (b)-(i), (c)-(ii), (d)-(iv)
(c) (a)-(ii), (b)-(iii), (c)-(iv), (d)-(i)
(d) (a)-(iii), (b)-(ii), (c)-(i), (d)-(iv)
Ans: (d)

Q2: Given below are two statements : one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A) : The metal carbon bond in metal carbonyls possesses both σ and π character.
Reason (R) : The ligand to metal bond is a π bond and metal to ligand bond is a σ bond.
In the light of the above statements, choose the most appropriate answer from the options given below.
(a) (A) is not correct but (R) is correct
(b) Both (A) and (R) are correct and (R) is the correct explanation of (A)
(c) Both (A) and (R) are correct but (R) is not the correct explanation of (A)
(d) (A) is correct but (R) is not correct (NEET 2022 Phase 2)
Ans: (d)

In case of metal carbonyls, the bonding has both σ and π nature, where ligand to metal bond is 'σ ' (coordinate) bond and metal to ligand bond is 'π' (synergic) bond.
Q3: The IUPAC name of the complex- [Ag(H2O)2][Ag(CN)2] is: (NEET 2022 Phase 1)
(a) dicyanidosilver(I) diaquaargentate(I)
(b) diaquasilver(I) dicyanidoargentate(I)
(c) dicyanidosilver(II) diaquaargentate(II)
(d) diaquasilver(II) dicyanidoargentate(II)
Ans: (b)
[Ag(H2O)2][Ag(CN)2]
IUPAC name : diaquasilver(I) dicyanidoargentate(I)
Q4: The order of energy absorbed which is responsible for the color of complexes (NEET 2022 Phase 1)
(A) [Ni(H2O)2(en)2]2+
(B) [Ni(H2O)4(en)2]2+
(C) [Ni(en)3]2+
(a) (C) > (A) > (B)
(b) (B) > (A) > (C)
(c) (A) > (B) > (C)
(d) (C) > (B) > (A)
Ans: (a)
Stronger the field strength of ligand, higher will be the energy absorbed by the complex.
'en' has a stronger field strength than 'H2O' according to spectrochemical series
Correct order of energy absorbed will be :
[Ni(en)3]2+ > [Ni(H2O)2(en)2]2+ > [Ni(H2O)4(en)]2+
i.e. (C) > (A) > (B)
2021
Q1: Ethylene diaminetetraacetate (EDTA) ion is: (NEET 2021)
(a) Bidentate ligand with two "N" donor atoms
(b) Tridentate ligand with three "N" donor
(c) Hexadentate ligand with four "O" and two atoms "N'' donor atoms
(d) Unidentate ligand
Ans: (c)
Ethylene diaminetetraacetate (EDTA) ion is a hexadented ligand having four donor oxygen atoms and two donor nitrogen atoms

Q2: Match List-I with List-II : (NEET 2021)

Choose the correct answer from the options given below.
(a) (a)-(iv), (b)-(i), (c)-(ii), (d)-(iii)
(b) (a)-(iv), (b)-(ii), (c)-(i), (d)-(iii)
(c) (a)-(ii), (b)-(iv), (c)-(iii), (d)-(i)
(d) (a)-(i), (b)-(iii), (c)-(iv), (d)-(ii)
Ans: (c)
Magnetic moment,  BM (where n = number of unpaired electrons)
BM (where n = number of unpaired electrons)

2020
Q1: The calculated spin only magnetic moment of Cr2+ ion is? (NEET 2020)
(a) 4.90 BM
(b) 5.92 BM
(c) 2.84 BM
(d) 3.87 BM
Ans: (a)
Electronic configuration of 
Electronic configuration of 
No. of unpaired electrons = 4
Spin only magnetic moment = 
n = number of unpaired e-
Spin only magnetic moment = 
= √24
= 4.9 BM
Q2: Urea reacts with water to form A which will decompose to form B. B when passed through Cu2+ (aq), deep blue colour solution C is formed. What is the formula of C from the following?
(a) 
(b) 
(c) 
(d) 
Ans: (a)

Q3: Which of the following is the correct order of increasing field strength of ligands to form coordination compounds? (NEET 2020)
(a) 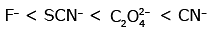
(b) 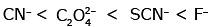
(c)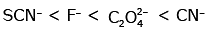
(d)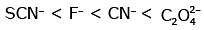
Ans: (c)
Fact from spectrochemical series.
2019
Q8. What is the correct electronic configuration of the central atom in K4[Fe(CN)6] based on crystal field theory? (NEET 2019)
(a)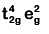
(b)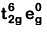
(c)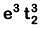
(d)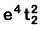
Ans: (b)
K4[Fe(CN)6]
Fe ground state: [Ar]3d64s2
Fe2+: 3d64s0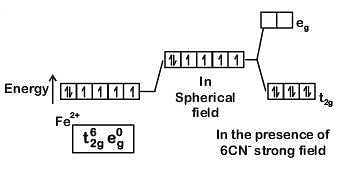
2018
Q1: The geometry and magnetic behaviour of the complex [Ni(CO)4] are (NEET 2018)
(a) square planar geometry and diamagnetic
(b) tetrahedral geometry and diamagnetic
(c) square planar geometry and paramagnetic
(d) tetrahedral geometry and paramagnetic
Ans: (b)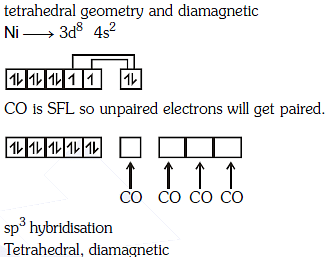
Q2: Iron carbonyl, Fe(CO)5 is (NEET 2018)
(a) Tetranuclear
(b) Mononuclear
(c) Trinuclear
(d) Dinuclear
Ans: (b)
Based on the number of metal atoms present in a complex, they are classified into mononuclear, dinuclear, trinuclear and so on. e.g. :
Fe(CO)5 : mononuclear
Co2(CO)8 : dinuclear
Fe3(CO)12 : trinuclear
Q3: The type of isomerism shown by the complex [CoCl2(en)2] is (NEET 2018)
(a) Geometrical isomerism
(b) Coordination isomerism
(c) Ionization isomerism
(d) Linkage isomerism
Ans: (a)
[CoCl2(en)2] shows geometrical isomerism and exist in cis and trans form. 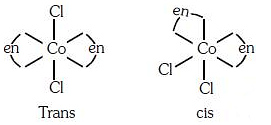
2017
Q1: An example of a sigma bonded organometallic compound is : (NEET 2017)
(a) Grignard's reagent
(b) Ferrocene
(c) Cobaltocene
(d) Ruthenocene
Ans: (a)
Grignard's reagent is an organometallic compound in which carbon atom is bonded to metal ion with sigma bond while in ruthenocene, ferrocene and cobaltocene are the organo metallic compounds in which carbon atom is attached to metal ion thorough π-bonding.
Q2: The correct order of the stoichiometries of AgCl formed when AgNO3 in excess is treated with the complexes : CoCl3.6NH3, CoCl3.5NH3,CoCl3.4NH3 respectively is :- (NEET 2017)
(a)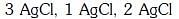
(b)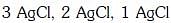
(c)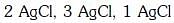
(d)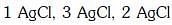
Ans: B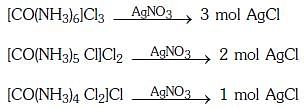
Q3: Correct increasing order for the wavelengths of absorption in the visible region the complexes of Co3+ is :- (NEET 2017)
(a)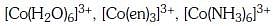
(b)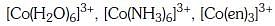
(c)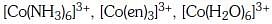
(d)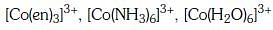
Ans: D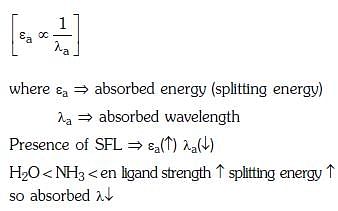
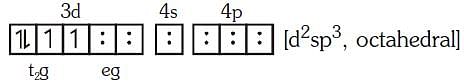
Q4: Pick out the correct statement with respect to[Mn(CN)6]3–:- (NEET 2017)
(a) It is sp3d2 hybridised and tetrahedral
(b) It is d2sp3 hybridised and octahedral
(c) It is dsp2 hybridised and square planar
(d) It is sp3d2 hybridised and octahedral
Ans: B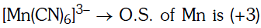
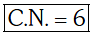

2016
Q1: Jahn-Teller effect is not observed in high spin complexes of (NEET 2016 Phase 2)
(a) d7
(b) d8
(c) d4
(d) d9
Ans: (b)
- Jahn-Teller distortion is generally significant for asymmetrically occupied eg orbitals as they are directed towards the ligands and the energy gain is more.
- On the other hand in unevenly occupied t2g orbitals, the John-Teller distortion is very weak. Since the t2g orbitals does not point directly at the ligand and thus energy gain is less.

Q2: The correct increasing order of trans-effect of the following species is (NEET 2016 Phase 2)
(a) 
(b) 
(d) 
(d) 
Ans: (b)
The intensity of the trans-effect as measured by the increase in rate of substitution of the trans ligand) follows the sequence :
CN
Q3: Which of the following has longest C-O bond length ? (Free C-O bond length in CO is 1.128Å) (NEET 2016 Phase 1)
(a) [Mn(CO)6]+
(b) Ni(CO)4
(c) [Co(CO)4]-
(d) [Fe(CO)4]2-
Ans: (d)
The greater the negative charge on the carbonyl complex, the more easy it would be for the metal to permit its electrons to participate in the back bonding, the higher would be the M-C bond order and simultaneously there would be larger reduction in the C-O bond order. Thus, [Fe(CO)4]2- has the lowest C-O bond order means the longest bond length.
2015
Q1: Which of these statements about [Co(CN)6]3- is true ? (AIPMT 2015 Cancelled Paper)
(a) [Co(CN)6]3- has no unpaired electrons and will be in a high-spin configuration
(b) [Co(CN)6]3- has no unpaired electrons and will be in a low-spin configuration
(c) [Co(CN)6]3- has four unpaired electrons and will be in a low-spin configuration.
(d) [Co(CN)6]3- has four unpaired electrons and will be in a high-spin configuration
Ans: (b)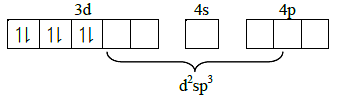
[Co(CN)6]3- has no unpaired electrons and will be in a low-spin configuration.
Q2: Cobalt(III) chloride forms several octahedral complexes with ammonia. Which of the following will not give test for chloride ions with silver nitrate at 25°C ? (AIPMT 2015 Cancelled Paper)
(a) CoCl3.6NH3
(b) CoCl3.3NH3
(c) CoCl3.4NH3
(d) CoCl3.5NH3
Ans: (b)
a) [Co(NH3)3Cl3] → [Co(NH3)2Cl3]
b) [Co(NH3)4Cl2]Cl → [Co(NH3)4Cl2]+ +Cl-
c) [Co(NH3)5Cl]Cl2 → [Co(NH3)5Cl]+ +2Cl-
d) [Co(NH3)6Cl]Cl2 → [Co(NH3)6]+ +3Cl-
The compound (a) does not ionise So it does not give test for chloride ions.
2014
Q1: Among the following complexes the one which shows Zero crystal field stabilization energy (NEET 2014)
(CFSE) is :
(a) [Co(H2O)6]2+
(b) [Co(H2O)6]3+
(c) [Mn(H2O)6]3+
(d) [Fe(H2O)6]3+
Ans: D
Q2: Which of the following complexes is used to be as an anticancer agent? (NEET 2014)
A: cis − K2 [Pt Cl2 Br2]
(b) Na2CoCl4
(c) mer − [Co (NH3)3Cl3]
(d) cis − [Pt Cl2 (NH3)2]
Ans: D
cis-platin is known as an anticancer agent. The formula for cis-platin is cis-[PtCl2(NH3)2]. Here, the word cis refers to a cis geometrical isomer of [PtCl2(NH3)2].
|
108 videos|286 docs|123 tests
|
FAQs on NEET Previous Year Questions (2014-2024): Coordination Compounds - Chemistry Class 12
| 1. What are coordination compounds in chemistry? |  |
| 2. How do ligands coordinate with metal ions in coordination compounds? |  |
| 3. What is the coordination number of a metal ion in a coordination compound? |  |
| 4. How do coordination compounds exhibit different colors? |  |
| 5. What is the difference between coordination isomerism and linkage isomerism in coordination compounds? |  |

|
Explore Courses for NEET exam
|

|


















