NEET Previous Year Questions (2014-2024): Redox Reactions | Chemistry Class 11 PDF Download
2024
Q1: Which reaction is NOT a redox reaction?(a) Zn + CuSO4 → ZnSO4 + Cu
(b) 2KClO3 + I2 → 2KIO3 + Cl2
(c) H2 + Cl2 → 2HCl
(d) BaCl2 + Na2SO4 → BaSO4 + 2NaCl (NEET 2024)
Ans: (d)

This is not a redox reaction as there is no change in oxidation state.
Q2: The products A and B obtained in the following reactions, respectively, are
(a) 
(b) 
(c) 
(d)  (NEET 2024)
(NEET 2024)
Ans: (d)
Let's analyze the given reactions and determine the products A and B.
Reaction with PCl3:
When an alcohol (ROH) reacts with phosphorus trichloride (PCl3), the hydroxyl group (OH) of the alcohol is replaced by a chloro group (Cl), resulting in the formation of an alkyl chloride (RCl). Additionally, the reaction produces phosphorous acid as a byproduct. This can be represented as:
3ROH + PCl3 → 3RCl + H3PO3
Here, A is H3PO3 (phosphorous acid).
Reaction with PCl5:
When an alcohol reacts with phosphorus pentachloride (PCl5), it also replaces the hydroxyl group with a chloro group. The reaction differs
slightly in the byproducts; phosphorus oxychloride (POCl3) and hydrogen chloride (HCl) are produced:
ROH + PCl5 → RCl + HCl + POCl3
Here, B is POCl3 (phosphorus oxychloride).
Thus, in the reactions provided:
A = H3PO3
B = POCl3
Matching these findings against the provided options, we see that Option D correctly identifies A as H3PO3 and B as POCl3:
Option D: H3PO3 and POCl3
This is the correct answer.
2023
Q1: On balancing the given redox reaction, (NEET 2023)
the coefficients a, b and c are found to be, respectively-
(a) 1, 3, 8
(b) 3, 8, 1
(c) 1, 8, 3
(d) 8, 1, 3
Ans: (a)
Using Ion electron method: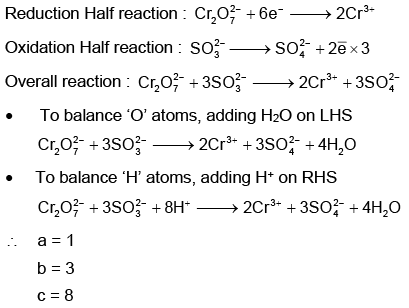
 Balancing Redox Reactions by Oxidation Number Method
Balancing Redox Reactions by Oxidation Number Method
2022
Q1: Which of the following reactions is a decomposition redox reaction? (NEET 2022 Phase 2)
(a) P4(s) + 3OH
(b) 2Pb(NO3)2(s)
(c) N2(g) + O2(g)
(d) Cl2(g) + 2OH
Ans: (b)
Decomposition redox reaction leads to breakdown of a compound into two or more compounds at least one of which must be in the elemental state with change in oxidation number.
2020
Q1: What is the change in oxidation number of carbon in the following reaction?
CH4(g) + 4Cl2(g)
(a) 0 to + 4
(b) −4 to + 4
(c) 0 to − 4
(d) + 4 to + 4
Ans: (b)

Change in oxidation state of carbon is from − 4 to + 4
2019
Q1: Which of the following reactions are disproportionation reaction? (NEET 2019)
(a) 2Cu+ → Cu2+ + Cu
(b) 
(c) 
(d) 
Select the correct option from the following
(a) (a) and (b) only
(b) (a), (b) and (c)
(c) (a), (c) and (d)
(d) (a) and (d) only
Ans: (a)
In a disproportionation reaction, same substance undergoes oxidation (increase in oxidation number) and reduction (decrease in oxidation number forming two different products.

2018
Q1: The correct order of N-compounds in its decreasing order of oxidation states is (NEET 2018)
(a) HNO3, NO, N2, NH4Cl
(b) HNO3, NO, NH4Cl, N2
(c) HNO3, NH4Cl, NO, N2
(d) NH4Cl, N2, NO, HNO3
Ans: (a)
Q2: For the redox reaction
MnO4– + C2O42–+ H+ →Mn2+ + CO2 + H2O
the correct coefficients of the reactants for the balanced equation are (NEET 2018)
MnO4– C2O4 2– H+
(1) 16 5 2
(2) 2 5 16
(3) 2 16 5
(4) 5 16 2
(a) 1
(b) 2
(c) 3
(d) 4
Ans: (b)
The correct balanced equation is
2MnO4– + 5C2O4 2– + 16H+
2016
Q1: Hot concentrated sulphate acid is a moderately strong oxidizing agent. Which of the following reactions does not show oxidizing behaviour?
(a) Cu + 2H2SO4
(b) S + 2H2SO4
(c) C + 2H2SO4
(d) CaF2 + H2SO4
Ans: (d)
CaF2 + H2SO4
Here, the oxidation state of every atom remains the same so, it is not a redox reaction.
2014
Q1: The pair of compounds that can exist together is : (NEET 2014)
(a) HgCl2 ,SnCl2
(b) FeCl2 ,KI
(c) FeCl3,SnCl2
(d) FeCl2 ,SnCl2
Ans: (a)
The compounds with lower oxidation number and which cannot reduce by one another can exist together. Thus, FeCl2 and SnCl2 can exist together as Fe2+ can not be reduced by Sn2+.
Q2: In acidic medium H2O2 changes Cr2O7 to CrO5 which has two −O−O− bonds. Oxidation state of Cr in CrO5 is : (2014)
(a) +6
(b) -10
(c) +5
(d) +3
Ans: (a)
CrO5 has butterfly structure having two peroxo bonds.
Peroxo oxygen has -1 oxidation state.
Let oxidation state of Cr be 'x'
CrO5 : x + 4(-1) + 1 (-2) = 0 ⇒ x = + 6
|
129 videos|244 docs|88 tests
|
FAQs on NEET Previous Year Questions (2014-2024): Redox Reactions - Chemistry Class 11
| 1. What is a redox reaction? |  |
| 2. How do you determine if a reaction is a redox reaction? |  |
| 3. What is the role of oxidizing and reducing agents in a redox reaction? |  |
| 4. Can you give an example of a redox reaction? |  |
| 5. How are redox reactions important in everyday life? |  |

|
Explore Courses for NEET exam
|

|













