NEET Previous Year Questions (2014-2024): States of Matter (Old NCERT) | Chemistry Class 11 PDF Download
Q.1. Which amongst the following options is correct graphical representation of Boyle’s law? (2023)
(a) 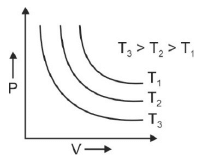
(b) 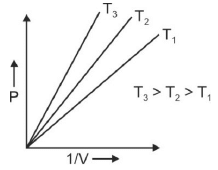
(c) 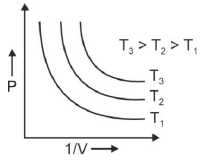
(d) 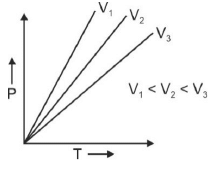
Ans: b
According to Boyle’s law,
PV = nRT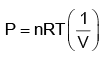
P versus (1/V) gives straight line graph with slope nRT.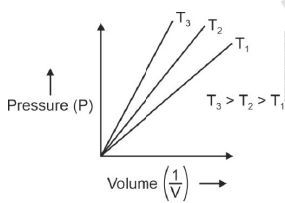
Q.2. Intermolecular forces are forces of attraction and repulsion between interacting particles that will include: (2023)
A. dipole - dipole forces
B. dipole - induced dipole forces
C. hydrogen bonding
D. covalent bonding
E. dispersion forces
Choose the most appropriate answer from the options given below:
(a) B, C, D, E are correct
(b) A, B, C, D are correct
(c) A, B, C, E are correct
(d) A, C, D, E are correct
Ans: c
Intermolecular forces are the forces of attraction and repulsion between interacting molecules. This term does not include covalent bonds as covalent bond holds atoms of a molecule together. Hence, dipole - dipole forces, dipole - induced dipole forces, hydrogen bonding and dispersion forces are intermolecular forces.
Q.3. Which one is not correct mathematical equation for Dalton's Law of partial pressure? Here p = total pressure of gaseous mixture (2022)
(a) pi = xip, where pi = partial pressure of ith gas
xi = mole fraction of ith gas in gaseous mixture
(b) pi = xi, pio where xi = mole fraction of ith gas in gaseous mixture
pio = pressure of ith gas in pure state
(c) p = p1 + p2 + p3
(d) 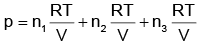
Ans: b
pi = xi poi is mathematical form of Raoult’s law rest all represents Dalton’s law
i.e.
(i) pi = xi pTotal
(ii) ptotal = p1 + p2 + p3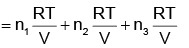
Q.4. A 10.0 L flask contains 64 g of oxygen at 27°C. (Assume O2 gas is behaving ideally). The pressure inside the flask in bar is (Given R = 0.0831 L bar K–1 mol–1) (2022)
(a) 49.8
(b) 4.9
(c) 2.5
(d) 498.6
Ans: b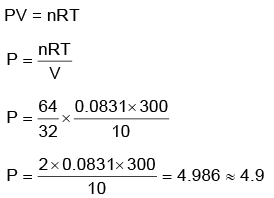
Q.5. The volume occupied by the molecules contained in 4.5 kg water at STP, if the intermolecular forces vanish away is (2022)
(a) 5.6 × 103 m3
(b) 5.6 × 10–3 m3
(c) 5.6 m3
(d) 5.6 × 106 m3
Ans: c
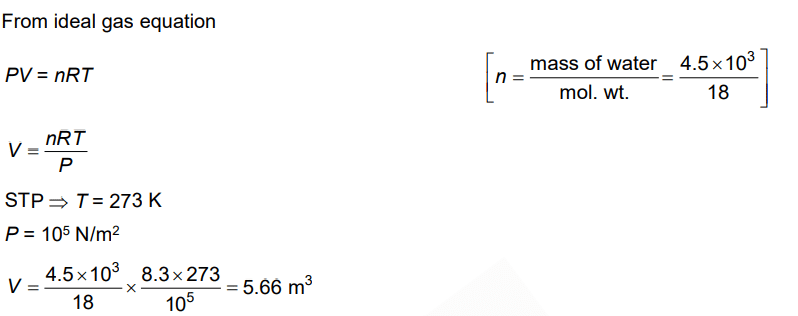
Q.6. Choose the correct option for graphical representation of Boyle's law, which shows a graph of pressure vs. volume of a gas at different temperatures : (2021)
(a) 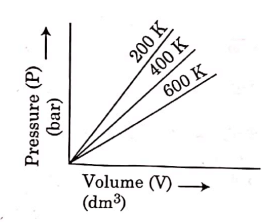
(b) 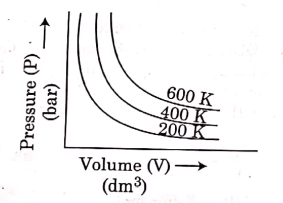
(c) 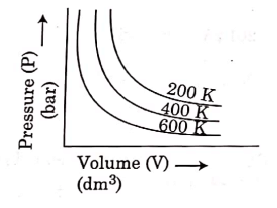
(d) 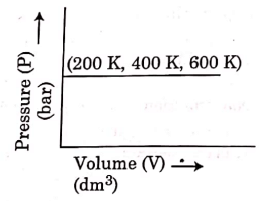
Ans: b
Hint: Boyle's Law
At constant temperature, the pressure of a fixed amount (i.e., number of moles n) of gas varies inversely with its volume.
p ∝ 1/v
The graph between p and V is as follows: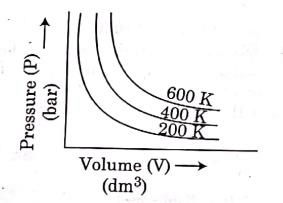
As temperature increases, pressure increases because the pressure is the directly proportional volume of gas.
Q.7. Choose the correct option for the total pressure (in atm.) in a mixture of 4 g O2 and 2 g H2 confined in a total volume of one litre at 0°°C is :
[Given R=0.082 L atm mol-1 K-1, T=273 K] (2021)
(a) 25.18
(b) 26.02
(c) 2.518
(d) 2.602
Ans: a
Hint: PV = nRT
Step 1:
The given values are as follows:
The amount of O2 is 4 g and the amount of H2 is 2 g. Calculate the number of moles of O2 and H2 is as follows: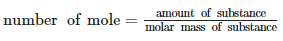
number of mole O2 = 4/32
number of mole O2 = 0.125 mol
number of mole H2 = 2/2
number of mole H2 = 1 mol
Step 2:
Calculate the value of total pressure is as follows:
P× 1 = 1.125 × 0.0821 × 273
P = 25.18 atm
Q.8. A mixture of N2 and Ar gases in a cylinder contains 7 g of N2 and 8 g of Ar. If the total pressure of the mixture of the gases in the cylinder is 27 bar, the partial pressure of N2 is: (2020)
[Use atomic masses (in g mol-1): N = 14, Ar = 40]
(a) 15 bar
(b) 18 bar
(c) 9 bar
(d) 12 bar
Ans: a
According to Dalton’s law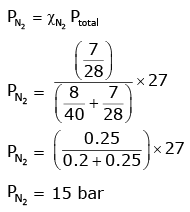
Q.9. A gas at 350 K and 15 bar has molar volume 20 percent smaller than that for an ideal gas under the same conditions. The correct option about the gas and its compressibility factor (Z) is : (2019)
(a) Z > 1 and attractive forces are dominant
(b) Z > 1 and repulsive forces are dominant
(c) Z < 1 and attractive forces are dominant
(d) Z < 1 and repulsive forces are dominant
Ans: c
Compressibility factor(Z) 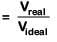
∵ Vreal < Videal; Hence Z < 1
If Z < 1, attractive forces are dominant among gaseous molecules and liquefaction of gas will be easy.
Q.10. Given vander Waals constant for NH3, H2 and CO2 are respectively 4.17, 0.244, 1.36 and 3.59, which one of the following gases is most easily liquefied? (2018)
(a) NH3
(b) H2
(c) O2
(d) CO2
Ans: a
Critical temperature ∝ vanderwaal constant(a) maximum "a" ⇒ gas with maximum TC ⇒ easiest liquification = NH3
Q.11. The correction factor 'a' to the ideal gas equation corresponds to (2018)
(a) density of the gas molecules
(b) volume of the gas molecules
(c) electric field present between the gas molecules
(d) forces of attraction between the gas molecules
Ans: d
Vanderwaal constant (a) ∝ forces of attraction.
Q.12. Equal moles of hydrogen and oxygen gases are placed in a container with a pin-hole through which both can escape. What fraction of the oxygen escapes in the time required for one-half of the hydrogen to escape ? (2016)
(a) 1/2
(b) 1/8
(c) 1/4
(d) 3/8
Ans: b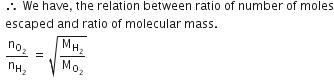
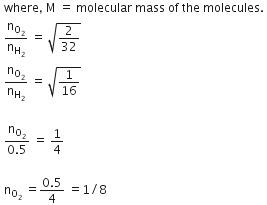
Q.13. Equal masses of H2, O2 and CH4 have been taken in a container of volume V at temperature 27° C in identical conditions. The ratio of the volumes of gases H2:O2:CH4 would be : (2014)
(a) 16 : 1 : 2
(b) 8 : 1 : 2
(c) 8 : 16 : 1
(d) 16 : 8 : 1
Ans: a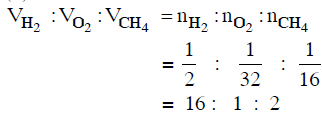
|
127 videos|245 docs|87 tests
|

|
Explore Courses for NEET exam
|

|

















