Nomenclature of Coordination Compounds | Chemistry Class 12 - NEET PDF Download
Why do we need to name the compounds?
How would your teacher call you and your best friend if you both didn’t have any names? Similarly, the naming of coordination compounds(complex nomenclature) is important to provide an unambiguous method to represent and describe formulas and names of coordination compounds systematically.
Also, it becomes very important while you deal with isomers. We follow a few rules of the International Union of Pure and Applied Chemistry (IUPAC) system while naming these compounds. In a nutshell, the rules are as follows:
In Coordination Compounds,
- within the coordination entities, we list down the central atom/ion first followed by the ligands.
- After this, we list down the ligands in alphabetical order.
- We write the formula of coordination entity in square brackets.
- Ligand abbreviations are to be enclosed in parentheses.
- We must not keep any space between the ligands and the metal within a coordination sphere.
- The charge on the cation(s) should be equal to the charge of the anion(s).
Now, let us look at these rules of complex nomenclature in greater details, along with suitable examples:
- Rule 1: While naming a coordination compound, we always name the cation before the anion. This rule does not count the fact of whether the complexion is cation or anion.
Example:
Na[Co(NH)4(Cl)2]: Na is to be named first followed by [Co(NH)4 (Cl)2], i.e. Sodium tetramminedichlorocoblatate(I)
[Co(NH)4(Cl)2]SO4: [Co(NH)4(Cl)2] is to be named first followed by SO4, i.e Tetraamminedichlorocobalt(0) sulphate. - Rule 2: When we see that there are multiple types of ligands present in any coordination compound, we name the ligands in alphabetic order after by the name of central metal atom/ion.
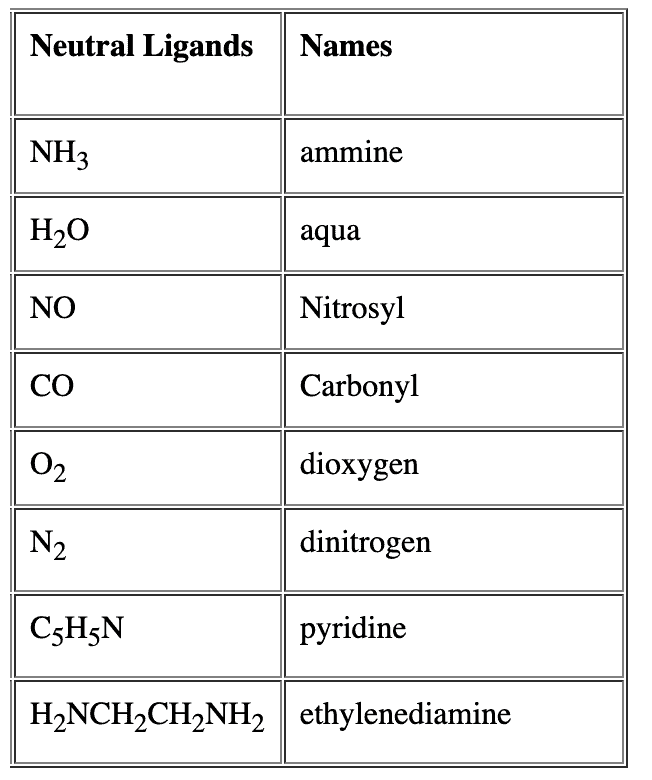
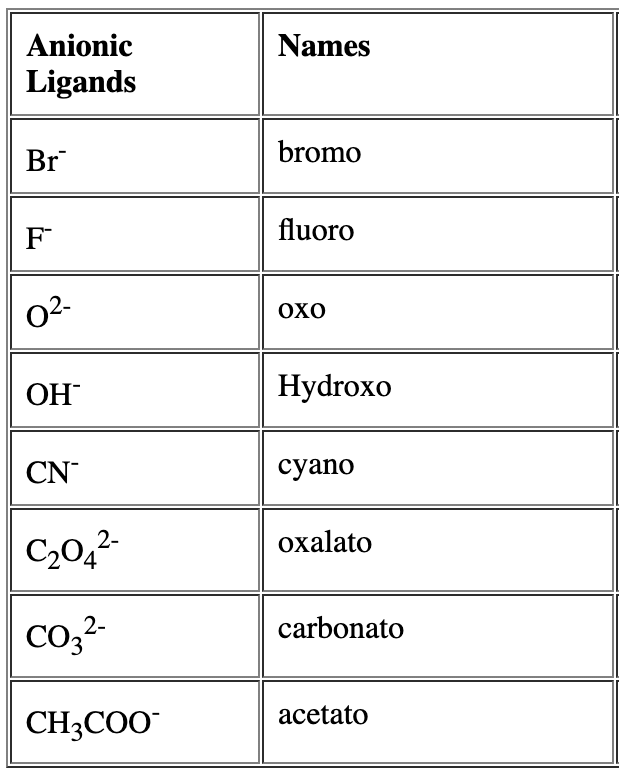
- Name of the anionic ligands ends with ‘o’. - For neutral ligands, their common name is used as such e.g.
- For neutral ligands, their common name is used as such e.g. Question for Nomenclature of Coordination CompoundsTry yourself:Identify the correct formula for hexaaquamanganese(II) ion.View Solution
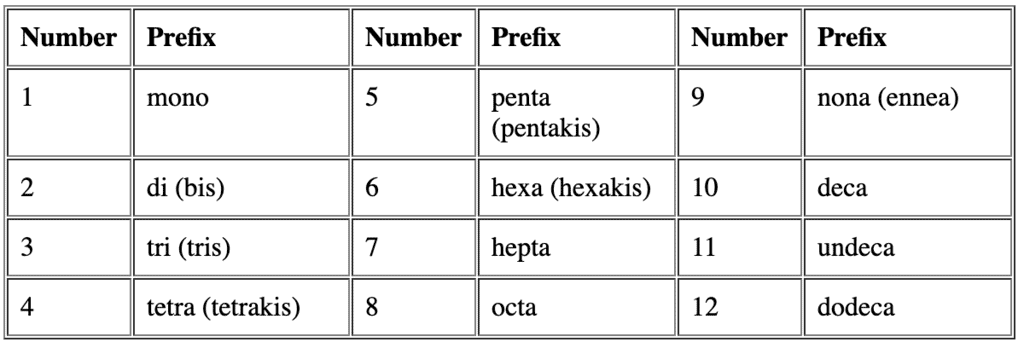
Question for Nomenclature of Coordination CompoundsTry yourself:Identify the correct formula for hexaaquamanganese(II) ion.View Solution - Rule 3: If the names of the ligands have a numerical prefix, then we use the terms bis, tris, tetrakis. This will represent the ligand to which they refer is placed in parentheses.
For example, we name [NiCl2(PPh3)2] as dichloridobis(triphenylphosphine)nickel(II)
- Rule 4: After naming the ligand in alphabetic order, we name the central metal atom/ion.
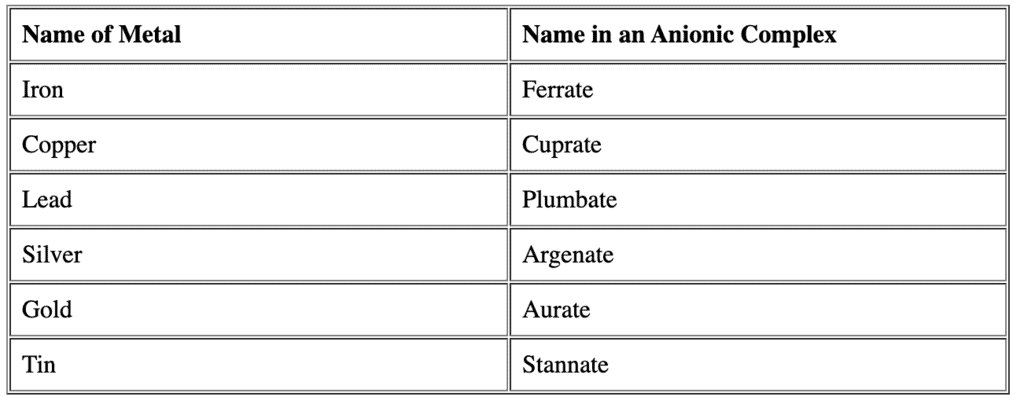
- If the complex ion is a cation, we name the metal the same as the element.
- If the complex is an anion, the name of metal ends with the suffix -ate for Latin name. Question for Nomenclature of Coordination CompoundsTry yourself:The correct way of naming Co in [CoCl2(en)2]+ is ________View Solution
Question for Nomenclature of Coordination CompoundsTry yourself:The correct way of naming Co in [CoCl2(en)2]+ is ________View Solution - Rule 5: We give the oxidation state of the metal in the complex as a roman numeral in parentheses.
- Rule 6: We name the neutral complex molecule similar to that of the complex cation.
- Rule 7: There are some ligands like NO2, CN that are attached to the central metal atom/ion through different atoms. We name them as follows:
M-NO2 → nitro
M-ONO → nitrito
M-SCN → thiocyanato
M-NCS → isothiocyanato
Rule 8: If any lattice component such as water or solvent of crystallisation is present, these follow their name, and are preceded by the number of these groups in Arabic numericals. These rules are illustrated by the following examples.
(a) Complex cations

IUPAC name

(b) Complex anions


(c) Organic groups


(d) Bridging groups


(e) Hydrates
AlK(SO4)2. 12H2O
Aluminium potassium sulphate 12-water Writing the formula of a coordination compound:
- When writing the formula of complexes, the complex ion should be enclosed by square brackets. The metal is named first, then the coordinated groups are listed in the order: negative ligands, neutral ligands, positive ligands (and alphabetically according to the first symbol within each group).
- [M negative ligands, Neutral ligands, positive ligands]

|
108 videos|286 docs|123 tests
|
FAQs on Nomenclature of Coordination Compounds - Chemistry Class 12 - NEET
| 1. Why is it important to name coordination compounds? |  |
| 2. What are the rules for naming coordination compounds? |  |
| 3. How does naming coordination compounds help in their identification? |  |
| 4. Are there any exceptions to the rules of naming coordination compounds? |  |
| 5. Can the same coordination compound have multiple names? |  |

|
Explore Courses for NEET exam
|

|
 - For neutral ligands, their common name is used as such e.g.
- For neutral ligands, their common name is used as such e.g.