Nomenclature of Organic Compounds | Chemistry Class 11 - NEET PDF Download

IUPAC Nomenclature
- IUPAC nomenclature of organic compounds refers to the systematic approach taken for the nomenclature of organic compounds as per the recommendation of the International Union of Pure and Applied Chemistry (often abbreviated to IUPAC).
- The necessity for such a systematic approach arose due to the sheer quantity of new discoveries of organic compounds which made the trivial nomenclature of organic compounds highly inconvenient.
However, the IUPAC nomenclature guidelines are not always followed by chemists since some compounds have very long and extremely tedious names as per the IUPAC nomenclature guidelines. These compounds are assigned more trivial names.

Nomenclature of Organic Compounds
- Organic chemistry deals with millions of compounds. In order to clearly identify them, a systematic method of naming has been developed and is known as the IUPAC (International Union of Pure and AppliedChemistry) system of nomenclature.
- In this systematic nomenclature, the names are correlated with the structure such that the reader or listener can deduce the structure from the name.
- Before the IUPAC system of nomenclature, however, organic compounds were assigned names based on their origin or certain properties.
- For instance, citric acid is named so because it is found in citrus fruits and the acid found in red ant is named formic acid since the Latin word for ant is Formica.

- These names are traditional and are considered trivial or common names. Some common names are followed even today.
- For example, Buckminsterfullerene is a common name given to the newly discovered C60 cluster (a form of carbon) noting its structural similarity to the geodesic domes popularized by the famous architect R. Buckminster Fuller.
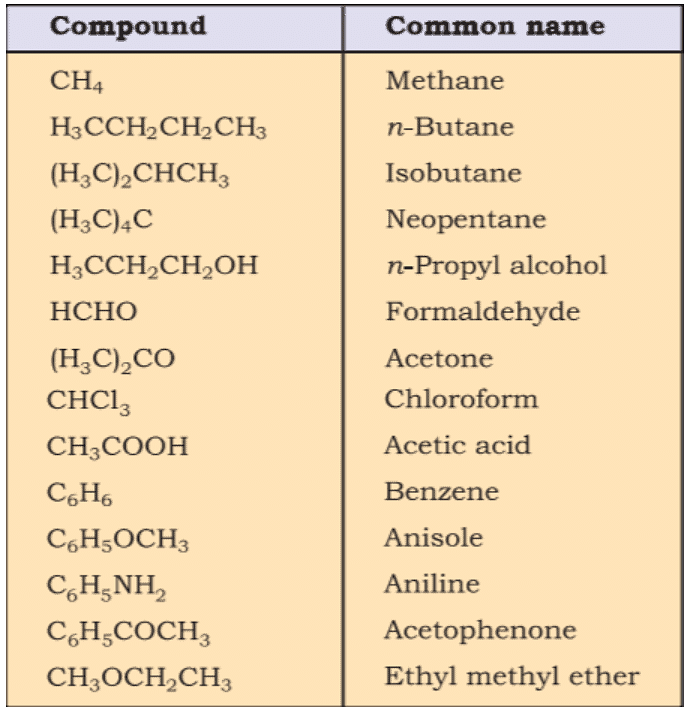
- Common names are useful and in many cases indispensable, particularly when the alternative systematic names are lengthy and complicated. Common names of some organic compounds are given in Table.
Common or Trivial Names of Some Organic Compounds
- A set of rules formulated by IUPAC (the International Union of Pure and Applied Chemistry) for systematic nomenclature of organic compounds is revised from time to time.
- The IUPAC nomenclature of organic compounds essentially consists of three parts which are Root Word, prefix and suffix.

- It is interesting to note that the existence of preferred IUPAC names does not prevent the use of other names to take into account a specific context or to emphasize structural features common to a series of compounds.
- Preferred IUPAC names belong to a “preferred IUPAC nomenclature”. Any name other than a preferred IUPAC name (as long as it is unambiguous and follows the principles of the IUPAC recommendations herein) is acceptable as a general IUPAC name in the context of a “general IUPAC nomenclature”.
The IUPAC System of Nomenclature
A systematic name of an organic compound is generally derived by identifying the parent hydrocarbon and the functional group(s) attached to it.

According to the Guidelines set by IUPAC, the nomenclature of compounds must follow these steps:
- The Longest Chain Rule: The parent hydrocarbon must be identified and subsequently named. The parent chain belonging to the compound in question is generally the longest chain of carbon atoms, be it in the form of a straight chain or a chain of any other shape.
- The Lowest Set of Locants: The carbon atoms belonging to the parent hydrocarbon chain must be numbered using natural numbers and beginning from the end in which the lowest number is assigned to the carbon atom which carries the substituents.
- Multiple instances of the same substituent: Prefixes which indicate the total number of the same substituent in the given organic compounds are given, such as di, tri, etc.
- Naming of different substituents: In the organic compounds containing multiple substituents, the corresponding substituents are arranged in alphabetical order of names in the IUPAC nomenclature of organic compounds in question.
- The naming of different substituents present at the same positions: In the scenario wherein two differing substituent groups are present at the same position of the organic compound, the substituents are named in ascending alphabetical order.
- Naming Complex Substituents: Complex substituents of organic compounds having branched structures must be named as substituted alkyl groups whereas the carbon which is attached to the substituent group is numbered as one. These branched and complex substituents must be written in brackets in the IUPAC nomenclature of the corresponding compounds.
- The format of the IUPAC Name of the Compound can be written as: Locant + Prefix + Root + Locant + Suffix

Root
The Word root indicates the total number of carbon atoms present in the longest carbon chain belonging to the compound. For example, ‘Meth’ refers to a chain with 1 carbon atom and ‘Pent’ refers to a chain with 5 carbon atoms.

Suffix
The suffix in IUPAC nomenclature is usually a functional group belonging to the molecule which follows the root of the name. It can be further divided into the following types.
 Primary Suffix, which is written immediately after the word root as in the case of alkanes, where the suffix is ‘ane’.
Primary Suffix, which is written immediately after the word root as in the case of alkanes, where the suffix is ‘ane’.
Secondary Suffix, Which is generally written after the primary suffix is written. For example, compounds having an alkane and alcohol group attached to them will be named alkanol, with ‘ol’ being the secondary suffix for the alcohol group.
In accordance with these norms, the suffix of a compound can be written as a part of the IUPAC name of the given compound.
Prefix
- Prefixes are added prior to the root of the compounds IUPAC nomenclature. Prefixes are very useful since they indicate the presence of side chains or substituent groups in the given organic molecule.
- These prefixes also offer insight into the cyclic or acyclic natures of the compounds in question.
- Primary Prefixes Indicate the cyclic or acyclic nature of the given compound. The prefix ‘cyclo’ is used for cyclic compounds.
- Secondary Prefixes Indicate the presence of side chains or substituent groups. An example of these types of prefixes would be the ‘CH3’ group, which is called the methyl group.
- Thus, prefixes in IUPAC nomenclature can be broadly classified into primary prefixes and secondary prefixes.
See the example given below.
 By further using prefixes and suffixes, the parent name can be modified to obtain the actual name.
By further using prefixes and suffixes, the parent name can be modified to obtain the actual name.- Compounds containing carbon and hydrogen only are called hydrocarbons. A hydrocarbon is termed saturated if it contains only carbon-carbon single bonds.
- The IUPAC name for a homologous series of such compounds is alkane.
- Paraffin (Latin: little affinity) was the earlier name given to these compounds. Unsaturated hydrocarbons are those, which contain at least one carbon-carbon double or triple bond.
IUPAC Nomenclature of Alkanes
Straight chain hydrocarbons: The names of such compounds are based on their chain structure, and end with suffix ‘-ane’ and carry a prefix indicating the number of carbon atoms present in the chain (except from CH4 to C4H10, where the prefixes are derived from trivial names). The IUPAC names of some straight-chain saturated hydrocarbons are given in Table. The alkanes in Table differ from each other by merely the number of -CH2 groups in the chain. They are homologues of alkane series.
IUPAC Names of Some Unbranched Saturated Hydrocarbons

Branched-chain hydrocarbons
In a branched-chain compound small chains of carbon atoms are attached at one or more carbon atoms of the parent chain. The small carbon chains (branches) are called alkyl groups. For example: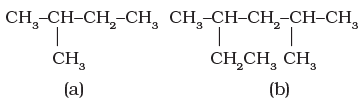
In order to name such compounds, the names of alkyl groups are prefixed to the name of parent alkane. An alkyl group is derived from a saturated hydrocarbon by removing a hydrogen atom from carbon. Thus, CH4 becomes -CH3 and is called methyl group. An alkyl group is named by substituting ‘yl’ for ‘ane’ in the corresponding alkane. Some alkyl groups are listed in Table.
 Abbreviations are used for some alkyl groups. For example, methyl is abbreviated as Me, ethyl as Et, propyl as Pr and butyl as Bu. The alkyl groups can be branched also.
Abbreviations are used for some alkyl groups. For example, methyl is abbreviated as Me, ethyl as Et, propyl as Pr and butyl as Bu. The alkyl groups can be branched also.
Thus, propyl and butyl groups can have branched structures as shown below.

Common branched groups have specific trivial names. For example, the propyl groups can either be n-propyl group or isopropyl group. The branched butyl groups are called sec-butyl, isobutyl and tert-butyl group. We also encounter the structural unit, –CH2C(CH3)3, which is called neopentyl group.
Three Basic Rules to remember for IUPAC naming
Rule I
Longest chain rule: The chain containing the principal functional group, secondary functional group and multiple bonds as many as possible is the longest possible chain.
In the absence of functional group, secondary group and multiple bonds, the chain containing the maximum number of C-atoms will be the longest possible chain e.g




Choose the word root from the table given below for the longest possible chain.
Word Root for Carbon Chain

Rule 2
Lowest number rule Numbering is done in such a way so that
1. branching if present gets the lowest number.
2. The sum of numbers of side chain is lowest.
3. The principal functional group gets the lowest number.
Select the principal functional group from the preference series :

Functional group other than the principal functional group are called substituents.
Rule 3
Naming the prefixes and suffixes Prefix represents the substituent and suffix are used for the principal functional group.
Primary prefixes are cycle, bicycle, di, tri, tetra, tetrakis etc.
Secondary prefixes are tabulated below :

Primary suffix are ene, ane, or yne used for double, single and triple bonds respectively.
Secondary suffixes are tabulated below :

Hence. according to the rules. given above, the IUPAC name of a compound can be written as:
Prefixes + Root word + Suffixes Primary prefix + secondary prefix + Root word + primary suffix + secondary suffix.

If more than two similar functional groups are present, all the groups are considered as substituent. e.g.,

Branched Chain Alkanes:
We encounter a number of branched chain alkanes. The rules for naming them are given below.
1. First of all, the longest carbon chain in the molecule is identified. In the example (I) given below, the longest chain has nine carbons and it is considered as the parent or root chain. Selection of parent chain as shown in (II) is not correct because it has only eight carbons.
2. The carbon atoms of the parent chain are numbered to identify the parent alkane and to locate the positions of the carbon atoms at which branching takes place due to the substitution of alkyl group in place of hydrogen atoms. The numbering is done in such a way that the branched carbon atoms get the lowest possible numbers.
Thus, the numbering in the above example should be from left to right (branching at carbon atoms 2 and 6) and not from right to left (giving numbers 4 and 8 to the carbon atoms at which branches are attached).
3. The names of alkyl groups attached as a branch are then prefixed to the name of the parent alkane and the position of the substituents is indicated by the appropriate numbers. If different alkyl groups are present, they are listed in alphabetical order. Thus, the name for the compound shown above is: 6-ethyl-2-methylnonane. [Note: the numbers are separated from the groups by hyphens and there is no break between methyl and nonane.]
4. If two or more identical substituent groups are present then the numbers are separated by commas. The names of identical substituents are not repeated, instead prefixes such as di (for 2), tri (for 3), tetra (for 4), penta (for 5), hexa (for 6) etc. are used. While writing the name of the substituents in alphabetical order, these prefixes, however, are not considered.
Thus, the following compounds are named as: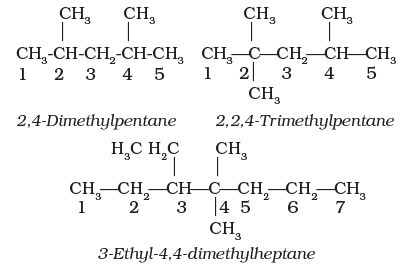
5. If the two substituents are found in equivalent positions, the lower number is given to the one coming first in the alphabetical listing. Thus, the following compound is 3-ethyl-6-methyloctane and not 6-ethyl-3-methyloctane.
6. The branched alkyl groups can be named by following the above mentioned procedures. However, the carbon atom of the branch that attaches to the root alkane is numbered 1 as exemplified below.


The name of such branched chain alkyl group is placed in parenthesis while naming the compound. While writing the trivial names of substituents’ in alphabetical order, the prefixes iso- and neo- are considered to be the part of the fundamental name of alkyl group. The prefixes sec- and tert- are not considered to be the part of the fundamental name. The use of iso and related common prefixes for naming alkyl groups is also allowed by the IUPAC nomenclature as long as these are not further substituted. In multisubstituted compounds, the following rules may aso be remembered:
- If there happens to be two chains of equal size, then that chain is to be selected which contains more number of side chains.
- After selection of the chain, numbering is to be done from the end closer to the substituent.

5-(2-Ethylbutyl)-3,3-dimethyldecane [and not 5-(2,2-Dimethylbutyl)-3-ethyldecane]
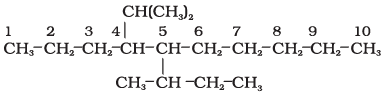
5-sec-Butyl-4-isopropyldecane
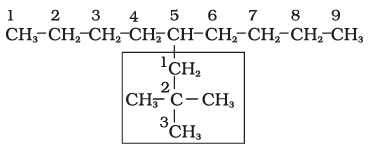
5-(2,2-Dimethylpropyl)nonane
Cyclic Compounds
A saturated monocyclic compound is named by prefixing ‘cyclo’ to the corresponding straight chain alkane. If side chains are present, then the rules given above are applied. Names of some cyclic compounds are given below.
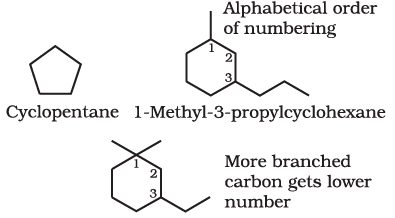
3-Ethyl-1,1-dimethylcyclohexane (not 1-ethyl-3,3-dimethylcyclohexane)
Alkenes
- The General formula of alkenes is described as CnH2n
- The suffix ‘ene’ is used to describe alkenes via IUPAC norms. Examples for the nomenclature of alkenes include the name ethene used to describe the compound given by C2H4 and Propene used to describe the compound given by C3H6

- Example: The IUPAC name of a molecule that contains a double bond between carbon atoms.
CH2=CH2: Eth + ene: Ethene
CH3–CH=CH2: Prop + ene: Propene
In writing the nomenclature of alkenes according to IUPAC, it is important to mention the position of double for the molecules which contain more than three carbon atoms.
CH2=CH–CH2–CH3:
Root word: But
Prefix: 1-ene
Root word + prefix: 1-Butene
CH3–CH=CH–CH3:
Root word: But
Prefix: 2-ene
Root word + prefix: 2-Butene
Alkynes
- The General formula of alkynes is CnH2n-2
- The suffix ‘yne’ is generally used to describe alkynes. An example of the IUPAC nomenclature of alkynes is: ethyne used to describe the compound given by C2H2
- The IUPAC name of a molecule contains triple bond between carbon atoms.
CH≡CH: Eth + yne: Ethyne
CH3–C≡CH: Prop + yne: Propyne
In writing nomenclature of alkynes according to IUPAC, it is important to mention the position of triple bond for the molecules which contain more than three carbon atoms.

CH≡C–CH2–CH2–CH3:
Root word: Pent
Prefix: 1-yne
Root word + prefix: 1-Pentyne
CH3–C≡C–CH2–CH3:
Root word: Pent
Prefix: 2–yne
Root word + prefix: 2-Pentyne
|
127 videos|244 docs|87 tests
|
FAQs on Nomenclature of Organic Compounds - Chemistry Class 11 - NEET
| 1. What is the importance of IUPAC nomenclature in organic chemistry? |  |
| 2. How do I determine the root name of an alkane? |  |
| 3. What are the basic rules for naming branched-chain hydrocarbons? |  |
| 4. What is the significance of prefixes and suffixes in IUPAC nomenclature? |  |
| 5. Can you explain the difference between common names and IUPAC names of organic compounds? |  |

|
Explore Courses for NEET exam
|

|



 By further using prefixes and suffixes, the parent name can be modified to obtain the actual name.
By further using prefixes and suffixes, the parent name can be modified to obtain the actual name.



















