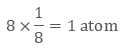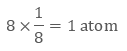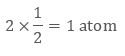NEET Exam > NEET Notes > Chemistry Class 12 > Number of Atoms in a Unit Cell
Number of Atoms in a Unit Cell | Chemistry Class 12 - NEET PDF Download
Introduction to Number of Atoms in a Unit Cell
That a crystal lattice comprises of several unit cells. In a unit cell, every constituent particle( atom, molecule or ion ) has a specific and fixed position called lattice site. We can calculate a number of atoms/molecules and ions in a unit cell easily by analyzing the nature and position of constituent particles in unit cells.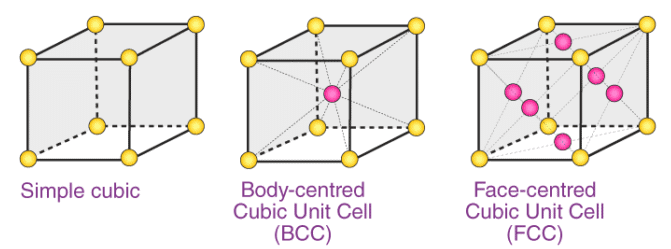
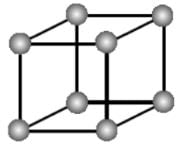
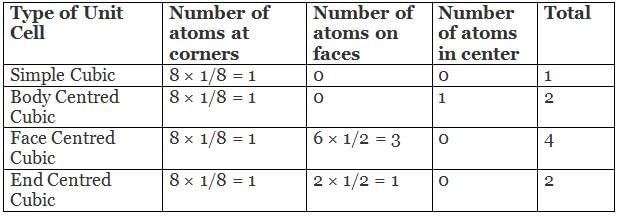
In the primitive cubic unit cell, the atoms are only located on the corners. That means 8 atoms are located on 8 corners of the lattice. Each atom located on the corner contributes 1/8th of the original volume of the cell. So since there are total 8 atoms in a primitive cubic
unit cell, the total number of atoms in the primitive cubic unit cell.
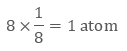
So there is only 1 atom in a primitive cubic unit cell.
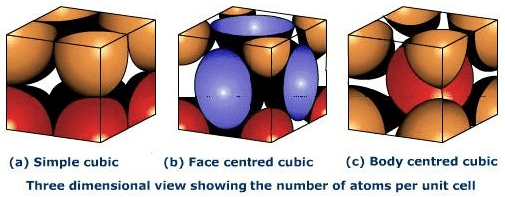
Image 2: The corners get only 1/8th part of atom(b) Body- centred Cubic Unit CellIn a body-centred unit cell, 8 atoms are located on the 8 corners and 1 atom is present at the center of the structure. So total atoms in the body-centred unit cell will be:Since 8 atoms are present at the corners, each will contribute 1/8th of the original volume of the cell. Thus in the body-centred cubic unit cell:
- There are 8 corners and 1 corner shares 1/8th volume of the entire cell, so

- Also, the atom at the centre is wholly present at the centre of the cell and can’t be shared
1 × 1 = 1 atom
So there are total 2 atoms present in a body centred unit cell.
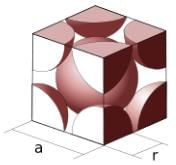
Image 3: Atoms in body-centrd unit cell
(c) Face-centred Cubic Unit Cell
In face-centred cubic unit cell atoms are present on 8 corners and center of all the faces. Also, each atom located on the centre of the unit cell is shared by two adjacent unit cells. Therefore only half atom belongs to a single unit cell.
Thus in Face-centred cubic unit cellIn face-centred cubic unit cell atoms are present on 8 corners and center of all the faces. Also, each atom located on the centre of the unit cell is shared by two adjacent unit cells. Therefore only half atom belongs to a single unit cell.
• There are 8 atoms present on 8 corners, therefore, each corner will get 1/8 part of atom
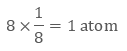
• There are six faces and each face gets 1/2 part of atom then
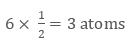
Total atoms present in a face-centred unit cell = 1 + 3 = 4 atoms
(d) End-centred Cubic Unit Cell
In end-centred cubic unit cell, 8 atoms are located on 8 corners of the cube and 1 atom each is present on two opposite faces of the cube.
Therefore in end-centred cubic unit cell
- There are 8 atoms present on 8 corners, therefore each atom contributes 1/8th portion of the cell

- There are 2 atoms located at the center of the cell and each atom contributes 1/2 portion of the cell

Total atoms present in a end-centred cubic unit cell = 1 + 1 = 2 atoms
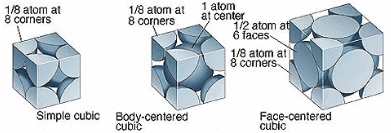
Image 4: Contribution of Atom in different unit cells
The table given below summarizes a total number of atoms present in a unit cell.
Image 5: Types of unit cells
The document Number of Atoms in a Unit Cell | Chemistry Class 12 - NEET is a part of the NEET Course Chemistry Class 12.
All you need of NEET at this link: NEET
|
108 videos|286 docs|123 tests
|
FAQs on Number of Atoms in a Unit Cell - Chemistry Class 12 - NEET
| 1. What is a unit cell? |  |
Ans. A unit cell is the smallest repeating structure within a crystal lattice. It represents the basic building block of a crystal and is used to describe the arrangement of atoms or ions in a crystal lattice.
| 2. How do you determine the number of atoms in a unit cell? |  |
Ans. To determine the number of atoms in a unit cell, you need to know the type of unit cell and the arrangement of atoms within it. Each type of unit cell has a specific number of atoms, which can be calculated using the crystal's structure and symmetry.
| 3. What factors affect the number of atoms in a unit cell? |  |
Ans. The number of atoms in a unit cell is primarily influenced by the crystal structure and the type of lattice. Different crystal structures, such as simple cubic, body-centered cubic, and face-centered cubic, have different numbers of atoms per unit cell.
| 4. How does the number of atoms in a unit cell affect the properties of a material? |  |
Ans. The number of atoms in a unit cell can significantly impact the properties of a material. It affects the density, atomic packing, and bonding characteristics, which, in turn, influence the material's mechanical, electrical, and thermal properties.
| 5. Can the number of atoms in a unit cell be fractional? |  |
Ans. No, the number of atoms in a unit cell cannot be fractional. It must be a whole number since a unit cell represents a repeating pattern within a crystal lattice. However, the fractional coordinates of atoms within a unit cell can be used to describe their positions relative to the unit cell's lattice points.

|
Explore Courses for NEET exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches