Order & Molecularity of a Reaction | Physical Chemistry for NEET PDF Download
Order of a Reaction
The order of a reaction refers to the power to which the concentration of a reactant is raised in the rate equation (rate law) for that particular reaction.
By performing a reaction in actual in laboratory and carefully examining it, it is possible to express the rate law as the product of concentrations of reactants each raised to some power.
For example consider the reaction : aA+ bB → cC +dD. The differential rate law is written as :
Rate =  =
=  =
=  =
=  = kr[A]m[B]n
= kr[A]m[B]n
where kr is called as rate constant of the reaction or velocity constant or specific Reaction rate.
k is a characteristic of a reaction at a given temperature. It changing only when the temperature changes.
The powers m and n are integers or fractions. m is called as order of reaction with respect to A and n is called as order of reaction with respect to B.The overall order of reaction = m n
Hence, the sum of powers of the concentration of the reactants in the rate law expression is called the order of that chemical reaction.
- The values of m and n are calculated from the experimental data obtained for a reaction and the powers m and n are not related to the stoichiometric coefficients of the reactants
- Order can be fractional, zero or negative.
For example consider the following reaction :
(i) H2(g) Br2(g) → 2 HBr (g) rate = k[H2] [Br2]1/2 (by experiment), order of reaction = 1 1/2 = 3/2
(ii) CH3CHO(g) → CH4(g) CO(g), rate = k[CH3CHO]3/2 , order of reaction = 3/2
Units of k:
In general, the rate law for a nth order reaction can be taken as :
where k : rate constant; c : concentration and n : order of reaction
⇒  ⇒ Units of k º (mol/L)1-n (time)-1
⇒ Units of k º (mol/L)1-n (time)-1
For a 'zero' order reaction (n = 0) : Units of k = (mol/L)1 (time)-1 or mol/L/sec
For a first order reaction (n = 1) : Units of k º (time)-1 e.g. sec-1, min-1, hrs-1 etc.
For a second order reaction (n = 2) : Units of k º (mol/L)-1 (time)-1 or L/mol/sec.
Molecularity of a Reaction
Molecularity refers to the number of molecules (or ions) that participate as reactants in an elementary reaction.
The number of reacting species (atoms, ions or molecules) taking part in an elementary reaction, which must collide simultaneously in order to bring about a chemical reaction is called molecularity of a reaction.
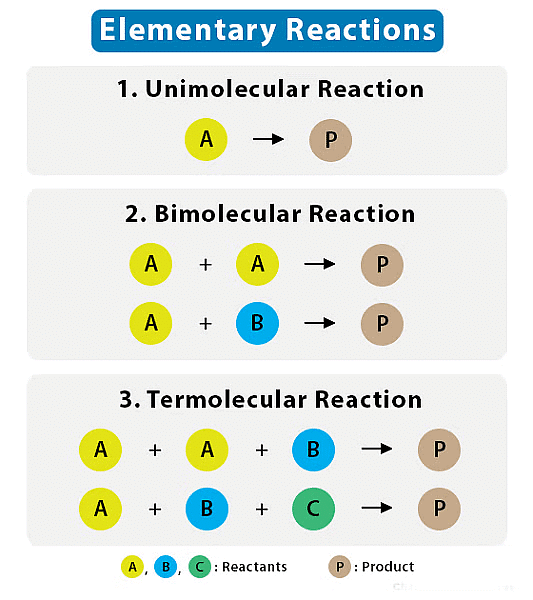
- Elementary reaction is a simple, single-step chemical reaction that involves a small number of reactant species and leads directly to the formation of products.
- Molecularity is a concept used specifically for elementary reactions, and it is not applicable to overall or complex reactions.
The molecularity of a reaction is often classified into three categories:
1. Unimolecular (Molecularity = 1)
- In a unimolecular reaction, a single molecule is involved in the elementary reaction. The rate equation for a unimolecular reaction is often expressed as: Rate=k[A]
- An example of a unimolecular reaction is a first-order reaction, where a single molecule undergoes a reaction, and the rate is directly proportional to its concentration.
- Cyclopropane → propene
- O3(g) → O2(g) + O(g)
- N2O5(g) → N2O4(g) +1/2O2(g)
2. Bimolecular (Molecularity = 2)
- In a bimolecular reaction, two molecules come together to react in a single step. The rate equation for a bimolecular reaction is often expressed as:
Rate=k[A][B]
- An example of a bimolecular reaction is a second-order reaction, where the rate is proportional to the product of the concentrations of two reactants.
1. NO(g) + O3 (g) → NO2(g)+ O2(g)
2. 2HI(g) → H2(g) I2(g)
3. Termolecular (Molecularity = 3)
- In a termolecular reaction, three molecules collide and react simultaneously in a single step. Termolecular reactions are relatively rare because they require the simultaneous collision of three reactant molecules, which is less likely to occur. They are more common in gas-phase reactions.
- Termolecular reactions are not as frequently encountered as unimolecular or bimolecular reactions.
It's important to note that molecularity is a theoretical concept used to describe elementary reactions. In more complex reactions, which involve multiple elementary steps, the reaction order and molecularity may not be the same. Overall reaction orders are determined experimentally, while molecularity is a concept used to describe the individual elementary steps of a reaction mechanism.
Difference Between Order and Molecularity of a Reaction

Question 1 : The rate of formation of NO(g) in the reaction NOBr(g)→ NO(g) Br2(g) is found to be 1.6 × 10-4 M/s. Find the rate of overall reaction rate and rate of consumption of NOBr.
Solution: We have :
1.6 × 10-4 M/s.
First write a balanced chemical equation. 2NOBr(g) → 2NO(g) Br2(g)
Now, Rate of overall reaction =
=
=
= 0.8 × 10-4 M/s
Rate of consumption of NOBr = -
= 1.6 × 10-4 M/s
Question 2 : The rate constant for a given reaction is k = 3 × 10-5 s-1 atm-1. Express it in units of L mol-1 sec-1.
Solution: PV = nRT ⇒ P = cRT (c : concentration in mol/L)
Substitute R = 0.0821 L-atm/mol/K ; T = 273 K ; P = 1 atm ⇒ c = 0.04462 mol/L
⇒
= 6.73 × 10-4 L/mol/s.
Question 3 : From the rate laws for the reactions given below, determine the order with respect to each species and the overall order.
(i) 2HCrO4- + 6I- + 14H → 2Cr3 + 3I2+8H2O, Rate = k[HCrO4-] [I-]2 [H ]2
(ii) H2O2 +2I- +2H → I2 +2H2O, Rate = k[H2O2] [I-]
Solution: (i) The order of the reaction with respect to [HCrO4-] is 1; with respect to [I-] is 2 and with respect to [H ] is 2. The overall order of the reaction is 1 +2 +2 = 5
(ii) The order of the reaction with respect to [H2O2] is 1 and with respect to [I-] is 1. The overall order of the reaction is 1 +1= 2.
- In (i) stoichiometric coefficient of I- is 6 whereas the power coefficient (n) in the rate law is 2.
- Reaction (i) may not take place in a single step. It may not be possible for all the 22 molecules to be in a state to collide with each other simultaneously. Such a reaction is called a complex reaction.
- A complex reaction takes place in a series of a number of elementary reactions.
|
117 videos|225 docs|239 tests
|
FAQs on Order & Molecularity of a Reaction - Physical Chemistry for NEET
| 1. What is the difference between order and molecularity of a reaction? |  |
| 2. How can the order of a reaction be determined experimentally? |  |
| 3. Can a reaction have a fractional order? |  |
| 4. What is the significance of determining the order of a reaction? |  |
| 5. How does the order of a reaction affect the rate constant? |  |

|
Explore Courses for NEET exam
|

|


















