Oxidation and Reduction of Aromatic Compounds | Chemistry Optional Notes for UPSC PDF Download
Oxidation of Alkyl Side-Chains
- The benzylic hydrogens of alkyl substituents on a benzene ring are activated toward free radical attack, as noted earlier. Furthermore, SN1 SN2 and E1 reactions of benzylic halides, show enhanced reactivity, due to the adjacent aromatic ring. The possibility that these observations reflect a general benzylic activation is supported by the susceptibility of alkyl side-chains to oxidative degradation, as shown in the following examples (the oxidized side chain is colored). Such oxidations are normally effected by hot acidic permanganate solutions, but for large scale industrial operations catalyzed air-oxidations are preferred. Interestingly, if the benzylic position is completely substituted this oxidative degradation does not occur (second equation, the substituted benzylic carbon is colored blue).

- These equations are not balanced. The permanganate oxidant is reduced, usually to Mn(IV) or Mn(II). Two other examples of this reaction are given below, and illustrate its usefulness in preparing substituted benzoic acids.

Bromination of the Benzylic Carbon
- The benzylic C-H bonds weaker than most sp3 hybridized C-H. This is because the radical formed from homolysis is resonance stabilized.

- Resonance stabilization of the benzylic radical

- Because of the weak C-H bonds, benzylic hydrogens can form benzylic halides under radical conditions.

NBS as a Bromine Source
NBS (N-bromosuccinimide) is the most commonly used reagent to produce low concentrations of bromine. When suspended in tetrachloride (CCl4), NBS reacts with trace amounts of HBr to produce a low enough concentration of bromine to facilitate the allylic bromination reaction.
Allylic Bromination Mechanism
- Step 1: Initiation: Once the pre-initiation step involving NBS produces small quantities of Br2, the bromine molecules are homolytically cleaved by light to produce bromine radicals.

- Step 2 and 3: Propagation

- Step 4: Termination

Solved Examples
Example 1: Predict the products of the following two reactions.
Ans: The second one leads to no reaction because it requires a hydrogen just off the phenyl ring.

Example 2: Consider a benzyl radical. Would it be more stable than an alkyl radical? Explain.
Ans: Yes, it would be more stable than an alkyl radical. The benzyl radical is stabilized through several resonance structures where the radical is moved through the ring via the pi system there.
Example 3: How would you make the following molecule? Ans: The following is just one possibility.
Ans: The following is just one possibility.
Aromatic Reduction Reactions
- Although it does so less readily than simple alkenes or dienes, benzene adds hydrogen at high pressure in the presence of Pt, Pd or Ni catalysts. The product is cyclohexane and the heat of reaction provides evidence of benzene's thermodynamic stability. Substituted benzene rings may also be reduced in this fashion, and hydroxy-substituted compounds, such as phenol, catechol and resorcinol, give carbonyl products resulting from the fast ketonization of intermediate enols. Nickel catalysts are often used for this purpose, as noted in the following equations.

- Benzene is more susceptible to radical addition reactions than to electrophilic addition. We have already noted that benzene does not react with chlorine or bromine in the absence of a catalyst and heat. In strong sunlight or with radical initiators benzene adds these halogens to give hexahalocyclohexanes. It is worth noting that these same conditions effect radical substitution of cyclohexane, the key factors in this change of behavior are the pi-bonds array in benzene, which permit addition, and the weaker C-H bonds in cyclohexane. The addition of chlorine is shown below on the left; two of the seven meso-stereoisomers are displayed to the right.

Reduction of Nitro Groups and Aryl Ketones
- Electrophilic nitration and Friedel-Crafts acylation reactions introduce deactivating, meta-directing substituents on an aromatic ring. The attached atoms are in a high oxidation state, and their reduction converts these electron withdrawing functions into electron donating amino and alkyl groups. Reduction is easily achieved either by catalytic hydrogenation (H2 + catalyst), or with reducing metals in acid.
- Examples of these reductions are shown here, equation 6 demonstrating the simultaneous reduction of both functions. Note that the butylbenzene product in equation 4 cannot be generated by direct Friedel-Crafts alkylation due to carbocation rearrangement. The zinc used in ketone reductions, such as 5, is usually activated by alloying with mercury (a process known as amalgamation).

- Several alternative methods for reducing nitro groups to amines are known. These include zinc or tin in dilute mineral acid, and sodium sulfide in ammonium hydroxide solution. The procedures described above are sufficient for most cases.
The Birch Reduction
Another way of adding hydrogen to the benzene ring is by treatment with the electron rich solution of alkali metals, usually lithium or sodium, in liquid ammonia. See examples of this reaction, which is called the Birch Reduction. The Birch reduction is the dissolving-metal reduction of aromatic rings in the presence of an alcohol.

Mechanism

Limitations of Friedel-Crafts Alkylation
- Carbocation Rearrangement: Only certain alkylbenzenes can be made due to the tendency of cations to rearrange.
- Compound Limitations: Friedel-Crafts fails when used with compounds such as nitrobenzene and other strong deactivating systems.
- Polyalkylation: Products of Friedel-Crafts are even more reactive than starting material. Alkyl groups produced in Friedel-Crafts Alkylation are electron-donating substituents meaning that the products are more susceptible to electrophilic attack than what we began with. For synthetic purposes, this is a big dissapointment.
To remedy these limitations, a new and improved reaction was devised: The Friedel-Crafts Acylation, also known as Friedel-Crafts Alkanoylation.
- The goal of the reaction is the following:

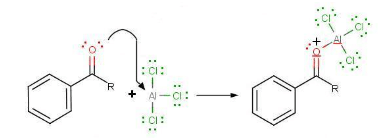
- The very first step involves the formation of the acylium ion which will later react with benzene:

- The second step involves the attack of the acylium ion on benzene as a new electrophile to form one complex:

- The third step involves the departure of the proton in order for aromaticity to return to benzene:

- During the third step, AlCl4 returns to remove a proton from the benzene ring, which enables the ring to return to aromaticity. In doing so, the original AlCl3 is regenerated for use again, along with HCl. Most importantly, we have the first part of the final product of the reaction, which is a ketone. Thie first part of the product is the complex with aluminum chloride as shown:

- The final step involves the addition of water to liberate the final product as the acylbenzene:

Because the acylium ion (as was shown in step one) is stabilized by resonance, no rearrangement occurs (Limitation 1). Also, because of of the deactivation of the product, it is no longer susceptible to electrophilic attack and hence, is no longer susceptible to electrophilic attack and hence, no longer goes into further reactions (Limitation 3). However, as not all is perfect, Limitation 2 still prevails where Friedel-Crafts Acylation fails with strong deactivating rings.
Solved Example
Example: How would you make the following from benzene and an acid chloride?
Ans:

|
Explore Courses for UPSC exam
|

|