Periodic Trends in Properties of Elements | Chemistry Class 11 - NEET PDF Download
Periodic Trends in Physical Properties of Elements
Effective Nuclear Charge:
- Between the outer most valence electrons and the nucleus of an atom, there exists finite number of shells containing electrons.
- Due to the presence of these intervening electrons, the valence electrons are unable to experience the attractive pull of the actual number of protons in the nucleus.
- These intervening electrons act as shield between the valence electrons and protons in the nucleus.
- Thus, the presence of intervening (shielding) electrons reduces the electrostatic attraction between the protons in the nucleus and the valence electrons because intervening electrons repel the valence electrons.
- The concept of effective nuclear charge allows us to account for the effects of shielding on periodic properties.
- The effective nuclear charge (Zeff) is the charge felt by the valence electron. Zeff is given by Zeff = Z – s. Where Z is the actual nuclear charge (atomic number of the element) and s is the shielding (screening) constant.
Atomic Radius:
- Probability of finding the electron is never zero even at large distance from the nucleus. Based on the probability concept, an atom does not have a well-defined boundary.
- Hence, exact value of the atomic radius can't be evaluated. Atomic radius is taken as the effective size which is the distance of the closet approach of one atom to another atom in a given bonding state.
Atomic radius can be
(A) Covalent radius:

It is one-half of the distance between the centres of two nuclei (of like atoms) bonded by a single covalent bond.
- Covalent radius is generally used for non-metals.
Single Bond Covalent Radius, SBCR -
(a) For Homoatomic moleucles 

- (b) For hetrodiatomic molecules in which electro negativity remains approximately same.

- For heteronuclear diatomic molecule, A – B, where difference between the electronegativity values of atom A and atom B is relativity larger,

where XA and XB electronegativity values of high electronegative element A and less electronegative element B, respectively. This formula is given by Stevenson & Schomaker.
Ex.2 Calculate the bond length of C – X bond, if C – C bond length is 1.54 Å, X – X bond length is 1.00 Å and electronegativity values of C and X are 2.0 and 3.0 respectively .
Sol. (1)
C – C bond length = 1.54 Å
rC = 154/100 = 0.77 Å
rX = 100/2 = 0.50 Å
(2) C – X bond length
dC–X = rC + rX – 0.09 (Xx – XC)
= 0.77 + 0.50 – 0.09 (3 – 2)
= 0.77 + 0.50 – 0.09 × 1
= 1.27 – 0.09 = 1.18 Å
Thus C – X bond length is 1.18 Å
(B) Van der Waals radius (Collision radius) :

It is one - half of the internuclear distance between two adjacent atoms in two nearest neighbouring molecules of the substance in solid state.
van der Waal's radius does not apply to metal. its magnitude depends upon the packing of the atoms when the element is in the solid state.
Comparision of convalent radius and van der Waal's radius
(i) The van der Waal's force of attractions are weak, therefore, their internuclear distances in case of atoms held by van der Waal's forces are much larger than those of between covalently bonded atoms.Therefore van der Waal's radii are always larger than covalent radii.
(ii) A covalent bond is formed by the overlaping of two half-filled atomic orbitals, a part of the orbital becomes common. Therefore, covalent radii are always smaller than the van der Waals radii. For example,
Element* | H | O | F | S | Br |
Covalent radius (A) | 0.37 | 0.66 | 0.64 | 1.04 | 1.11 |
vander Waal's radius (A) | 1,20 | 1.40 | 1.35 | 1.85 | 1.95 |
(C) Metallic radius (crystal radius) :

It is one -half of the distance between the nuclei of two adjacent metal atoms in the metallic crystal lattice. Metallic radius of an element is always greater than its covalent radius. It is due to the fact that metallic bond (electrical attraction between positive charge of an atom and mobile electrons) is weaker than covalent bond and hence the hence the internuclear distance between the two adjacent atoms in a metallic crystal is longer than the internuclear distance between the covalently bonded atom.
For example :
Metallic | radius | Covalent radius |
K | 231 pm | 203 pm |
Na | 186 pm | 154 pm |



Variation In a Period | Variation In a Group |
In a period left to right: | In a group top to bottom : |
Z increases by one unit | Z increases by more than one unit |
Zeff. also increases | Zeff. almost remains constant (due to increased screening effect of inner shells electrons) |
n remains constant (no of orbits) | n increases (no. of orbits) |
As a result of these electrons are pulled close to the nucleus by the increased Zeff. rn ∝ 1/Z* Thus atomic radii decreases with increase in atomic number in a period from left to right | The effect of increased number of atomic shells overweigh the effect of increased screening effect. |
- The atomic radius of inert gas (zero group) is given largest in a period because it is represented as van der Waals's radius which is generally larger than the covalent radius. The van der Waal's radius of inert gases also increases from top to bottom in a group.
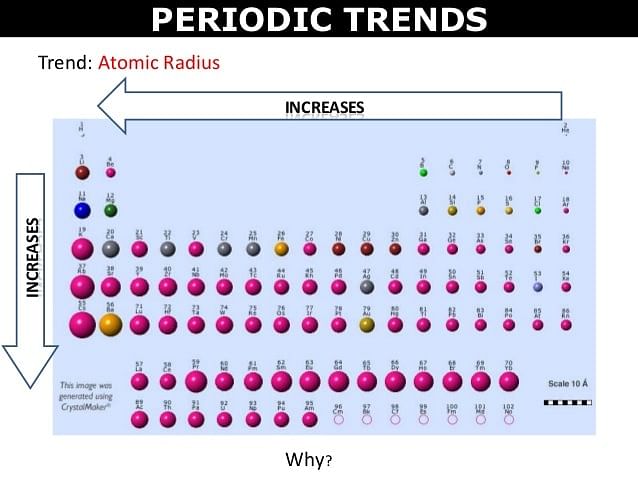
- In the transition series (e.g. in first transition series), the covalent radii of the elements decrease from left to right across a row until near the end when the size increases slightly. On moving from left to right, extra protons are placed in the nucleus and the extra electron are added. The orbital electrons shield the nucleus charge incompletely. Thus the nuclear charge attracts all the electrons more strongly, hence a contraction in size occurs. The radii of the elements from Cr to Cu, are very close to one another because the successive addition of d-electrons screen the outer electrons (4s) from the inward pull of the nucleus. As a result of this, the size of the atom does not change much in moving from Cr to Cu.

- The lanthanide contraction counter balances almost exactly the normal size increase on descending a group of transition elements. Thus covalent and ionic radii of Nb (5th peroid) and Ta (6th period) are almost same due to poor shielding of f-orbitals electrons.
Ionic Radius :
The effective distance from the centre of nucleus of the ion up to which it has an influence in the ionic bond is called ionic radius.
Cation | Anion |
It is formed by the toss of one or more electrons from the valence shell of an atom of an element. (ii) In a Cation, the number | It is formed by the gain of one or more electrons In the valence shell of an atom of an element. Anions are larger than the parent atoms because (1) Anion Is formed by gain of one or more electrons in the neutral atom and thus number of electron |
For example :
| Na | Na+ |
Number of Protons | 11 | 11 |
Electronic Configuration | 1s2 2s2 2p6 3s1 | 1s22s2 2p6 |
| Cl | Cl– |
Number of Electrons | 17 | 18 |
Number of Proton | 17 | 17 |
- The sizes of ions increases as we go down a group (cosidering the ions of same charge)
For example
Li+ < Na+ < K+ < Rb+ Be2+ < Mg2+ < Ca2+ < Sr2+ F– < Cl– < Br– < I– - The d and f orbitals do not shield the nuclear charge very effectively. Therefore there is significant reduction in the size of the ions, just after d or f orbitals have been filled completely. This is called a lanthanide contraction. Atomic radii of Zr and Hf are almost identical due to lanthanide contraction.
- The species containing the same number of electrons but differ in the magnitude of their nuclear charges are called as isoelectronic species. For example, N3- , O2-, F-, Ne, Na+ , Mg2+ and Al3+ are all isoelectronic species with same number of electrons (i.e, 10) but different nuclear charges of +7, +8, +9, +10, +11, +12 and +13 respectively.
Within a series of isoelectronic species as the nuclear charge increases, the force of attraction by the nucleus on the electrons also increases. As a result, the ionic radii of isoelectronic species decrease with increases in the magnitude of nuclear charges.
For example, 

- Pauling's empirical formula : Ionic radius

- Following are the examples of isoelectronic series (i) S2- Cl- K+ Ca+2 Sc+3 (ii) SO2, NO3–, CO32- (iii) N2, CO, CN– (iv) NH3, H3O–
Ionisation Enthalpy
lonisation enthalpy/energy (IE) , sometimes also called ionisation potential (IP) , of an element is defined as the amount of energy required to remove an electron from an isolated gaseous atom of that element resulting in the formation of positive ion.

IE2 & IE3 are the IInd & IIlrd ionization energies to remove electron from monovalent and divalent cations respectively.
In general: (IE)1 < (IE)2 < (IE)3 < .............
because, as the number of electrons decreases, the attraction between the nucleus'and the remaining electrons increases considerably and hence subsequent 1.E.(s) increase.
- Units of ionisation energy: KJ mol-1 ,K Cal mol-1, eV (electron volt)
- Factors Influencing lonisation enthalpy (IE) variation in a period and group may or may not be regular and can be influenced by:
(A) Size of the Atom : lonisation energy decreases with increase in atomic size. As the distance between the outermost electrons and the nucleus increases, the force of attraction between the valence shell electrons and the nucleus decreases. As a result, outer most electrons are held less firmly and lesser amount of energy is required to knock them out.
For example, ionisation energy decreases in a group from top to bottom with increase in atomic size.
(B) Nuclear Charge: The ionisation energy increases with an increase in the nuclear charge. This is due to the fact that with increase in the nuclear charge, the electrons of the outer most shell are more firmly held by the nucleus and thus greater amount of energy is required to pull out an electron from the atom. For example, ionisation energy increases as we move from left to right along a period due to increase in nuclear charge.
(C) Shielding effect: The electrons in the inner shells act as a screen or shield between the nucleus and the electrons in the outermost shell. This is called shielding effect. The larger the number of electrons in the inner shells, greater is the screening effect and smaller the force of attraction and thus (IE) decreases.

(D) Penetration Effect of the Electron : The ionisation energy increases as the penentration effect of the electrons increases.
It is a well known fact that the electrons of the s-orbital has the maximum probability of being found near the nucleus and this probability goes on decreasing in case of p, d and f orbitals of the same energy level. Within the same energy level, the penetration effect decreases in the order
s > p > d > f
Greater the penetration effect of electron more firmly the electron will be held by the nucleus and thus higher will be the ionisation energy of the atom.
For example, ionisation energy of aluminium is comparatively less than magnesium as outer most electron is to be removed from p-orbital (having less penetration effect) in aluminium where as in magnesium it will be removed from s-orbital (having large penetration effect) of same energy level.
(E) Electronic Configuration : If an atom has exactly half-filled or completely filled orbitals, then such an arrangement has extrastability.
The removal of an electron from such an atom requires more energy than expected. For example,
Be IE1 > B IE1 

As noble gases have completely filled electronic configuration, they have highest ionisation energies in their respective periods.




- Metallic or electropositive character of elements increases as the value of ionisation energy decreases.
- The relative reactivity of the metals increases with the decrease in ionisation energy.
- The reducing power of elements increases as the value of ionisation energy decreases (Li is exception in Alkali metals group which has highest reducing power)
Ex.3 First and second ionisation energies of Mg(g) are 740 and 1450 kJ mol–1. Calculate percentage of Mg+(g) and Mg2+(g), if 1 g of Mg(g) absorbs 50 kJ of energy.
Sol. Number of moles of 1g of Mg = 1/24 = 0.0417
Energy required to convert Mg(g) to Mg+(g) = 0.0417 x 740 = 30.83 kJ
Remaining energy = 50 – 30.83 = 19.17 kJ
Number of moles of Mg2+ formed = 17.19/1450 = 0.0132
Thus, remaining Mg+ will be = 0.0417 – 0.0132 = 0.0285
% Mg+ = (0.0285/ 0.0417) × 100 = 68.35%
% Mg+ = 100 – 68.35 = 31.65%
Electron Gain Enthalpy:
When an electron is added to a neutral gaseous atom (X) to convert it into a negative ion, the enthalpy change accompanying the process is defined as the electron gain enthalpy.
Electron gain enthalpy provides a measure of the ease with which an atom adds an electron to form anion.

Depending on the elements, the process of adding an electron to the atom can be either endothermic or exothermic. When an electron is added to the atom and the energy is released, the electron gain enthalpy is negative and when energy is needed to add an electron to the atom, the electron gain enthalpy is positive. The addition of second electron to an anion is opposed by electrostatic repulsion and hence the energy has to be supplied for the addition of second electron.

EA (i) is exothermic whereas EA(ii) is endothermic.
- Group 17 elements (halogens) have very high negative electron gain enthalpies because they can attain stable noble gas electronic configuration by picking up an electron.
- Noble gases have large positive electron gain enthalpies because the electron has to enter the next higher energy level leading to a very unstable electronic configuration.
- Electron gain enthalpy of O or F is less than S or CI. This is due to the fact that when an electron is added to O or F, the added electron goes to the smaller n = 2 energy level and experiences significant repulsion from the other electrons present in this level. In S or CI, the electron goes to the larger n = 3 energy level and consequently occupies a larger region of space leading to much less electron electron repulsion.
- Electron gain enthalpies of alkaline earth metals are very less or positive because the extra electron is to be added to completely filled s-orbitals in their valence shells.
- Across a period, with increase in atomic number, electron gain enthalpy becomes more negative because left to right across a period effective nuclear charge increases and consequently it will be easier to add an electron to a small atom.
- As we move in a group from top to bottom, electron gain enthalpy becomes less negative because the size of the atom increases and the added electron would be at larger distance from the nucleus.
(i) Electron affinity 
(ii) Electron affinity ∞ Effective nuclear charge (zeff)
(iii) Electron affinity
(iv) Stability of half filled and completely filled orbitals of a subshell is comparatively more and the addition of an extra electron to such an system is difficult and hence the electron affinity value decreases.
Ex.4 How many CI atoms can you ionise in the process  he energy liberated for the process
he energy liberated for the process  or one Avogadro number of atoms.
or one Avogadro number of atoms.
Given IP = 13.0 eVand EA= 3.60 eV
Sol. Let n atoms be ionised. 6.02 × 1023 × EA = n × IP

Ex.5 The first ionisation potential of Li is 5.4 eV and the electron affinity of CI is 3.6 eV Calculate Δ H in kcal mol–1 for the reaction.

Sol. The overall reaction is written into two partial equations

= 1.8 × 23.06 kcal mol–1
= 41.508 kcal mol–1
Ex.6 For the gaseous reaction,  was calculated to be 19 kcal under conditions where the cations and anions were prevented by electrosatic separation from combining with each other. The ionisation potential of K is 4.3 eV. What is the electron effinity of F ?
was calculated to be 19 kcal under conditions where the cations and anions were prevented by electrosatic separation from combining with each other. The ionisation potential of K is 4.3 eV. What is the electron effinity of F ?
Sol.

Ex.7 The electron affinity of chlorine is 3.7 eV. How much energy in kcal is released when 2 g of chlorine is completely converted to CI– ion in a gaseous state? (1 eV = 23.06 kcal mol-1)
Sol.

35.5 3.7 × 23.06 kcal
l .'. Energy released for conversion of 2 g gaseous chlorine into CI– ions
 × 2 = 4.8 kcal
× 2 = 4.8 kcal
Hydration Enthalpy
Hydration enthalpy, or hydration energy, is the energy that gets released when a gas ion mixes with water and becomes surrounded by water molecules, turning into a more stable form.
- Hydration enthalpy is the quantity of energy produced when 1 (one) mole of the gaseous ions is mixed with H2O (water) to produce hydrated ions.
- Hydration energy is a significant component in the brief analysis of solvation.
- One of the most challenging components of structural prediction is determining the number of hydration energies.
- When a salt is dissolved in water, anions and cations react with the water’s positive and negative dipoles.
The hydration enthalpy is proportional to the charge density of ions. The charge density of smaller ions is higher; hence the hydration enthalpy of smaller ions is higher. The attractive force between the ion and the polar end of water increases as the charge density increases. Thus, smaller ions have a more excellent hydration enthalpy value. The alkali metals have higher hydration enthalpy, and the degree of hydration diminishes as you move through the group in the periodic table.
- Hydration enthalpy, or hydration energy, depends on the size and charge of ions. It's the energy released when gaseous ions mix with water, forming stable hydrated ions surrounded by water molecules.
- Larger ions generally have lower hydration enthalpies, while smaller, more highly charged ions have higher enthalpies because they attract water molecules more strongly.
- For instance, Group 2 ions like Mg2+ have higher hydration enthalpies than Group 1 ions like Na+.
- As ions get larger down the periodic table, their hydration enthalpies decrease.
- This process is exothermic, meaning it releases energy, such as in reactions like cement mixing with water.
Note: Why does Hydration Enthalpy Decrease Down the Group?
Smaller the ion, the higher the hydration enthalpy will be because smaller atoms can accommodate a large number of water molecules around it and get hydrated. Hydration enthalpy decreases down the group; the size of the atom increases due to the addition of extra valence shells.
Also, the hydration enthalpy decreases since the size of the cation increases. However, due to the square factor, lattice enthalpy decreases faster than the hydration enthalpy. That's why the solubility of Group 2 hydroxides increases while it progresses down the group.
Lattice Enthalpy
The energy associated when are mole of an ionic crystal is formed from its gaseous ions is called Lattice energy.
- During the formation of solid ionic compounds, electropositive metals react with electronegative nonmetals.
- Both the generation and dissolution of such compounds involve the concept of lattice energy, a type of potential energy expressed in units of kJ/mol.
- Lattice energy maintains the fixed positions of cations and anions within ionic compounds.
- We can further investigate this term in two different ways, depending on our perspective.
- The key to understanding this concept lies in the crystalline structure of ionic compounds.
- Their strong, rigid composition enables interactions between each charged ion and its oppositely charged counterparts.
- These interactions involve large amounts of energy, explaining the high melting and boiling points characteristic of ionic compounds.
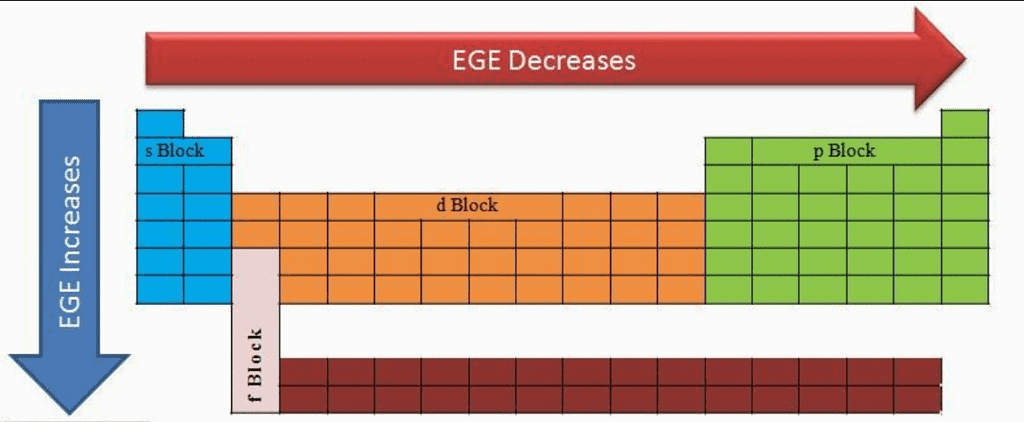
Electronegativity
Electronegativity is a measure of the tendency of an element to attract electrons towards itself in a covalently bonded molecules .
 The magnitude of electronegativity of an element depends upon its ionisation potential & electron affinity. Higher ionisation potential & electron affinity values indicate higher electronegativity value.
The magnitude of electronegativity of an element depends upon its ionisation potential & electron affinity. Higher ionisation potential & electron affinity values indicate higher electronegativity value.
- With increase in atomic size the distance between nucleus and valence shell electrons incerases, therefore, the force of attraction between the nucleus and the valence shell electrons decreases and hence the electronegativity values also decrease.
- With increase in nuclear charge force of attraction between nucleus and the valence shell electrons increases and, therefore, electronegativity value increases In higher oxidation state, the element has higher magnitude of positive charge. Thus, due to more positive charge on element, it has higher polarising power. Thus, with increase in the oxidation state of element, its electronegativity also increases.
- Charge on cation a electronegativity of the atom



There is no direct method to measure the value of electronegativity, however, there are some scales to measure its value .
(a) Pauling's Scale: Linus Pauling developed a method for calculating relative electronegativities of most elements. According to Pauling


(b) Mulliken's scale
Electronegativity (EN) can be regarded as the average ofthe ionisation energy (IE) and the electron affinity (EA) of an atom.

If both (EA) and (IE) are determined in eV units then paulings's electronegativity (EN)p is related to Mulliken's electronegativity. Mulliken's values were about 2.8 times larger than the Pauling's values.
(c) Allred-Rochow's Electronegatlvlty Allred and Rochow defined electronegativity as the force exerted by the nucleus of an atom on its valence electrons:

where Zelleclive is the effective nuclear charge and r the covalent radius (in Å ).
- The electron negativity of Cs (55) is less than Fr (87). This is due to the increase of + 32 units in nuclear charge of Fr which makes the effective nuclear charge comparatively high.
- The electroneativity of inert gas elements of zero group is zero. Inert gases exist as monoatomic molecules and the electronegativity is the property of bonded atoms.
Ex.8 lonisation potential and electron affinity of fluorine are 17.42 and 3.45 eV respectively. Calculate the electronegativity of fluorine.
Sol. According to Mulliken equation
 when both IP and EA are taken in eV..
when both IP and EA are taken in eV..
Applications of electronegativity :
(I) Nomenclature: Compounds formed from two nonmetals are called binary compounds. Name of more electronegative element is written at the end and 'ide' is suffixed to it. The name of less electronegative element is written before the name of more electronegative element of the formula.
Ex.9 Write the correct formula and name of the following (a) ICI or CIl (b) FCI or CIF (c) BrCI or CIBr (d) BrI or IBr (e)OF2 or F2O (f)Cl2O or OCI2
Sol. Correct formula Name
(a) I+ Cl– Iodine chloride
(b) CI+ F– Chlorine fluoride
(c) Br+ CI– Bromine chloride
(d) IBr Iodine bromide
(e) OF2 Oxygen difluoride
(f) Cl2O Dichlorine oxide
(II) Nature of Bond: If difference of electronegativities of the two elements is 1.7 or more, then ionic bond is formed between them whereas if it is less than 1.7, then covalent bond is formed. (HF is exception in which bond is covalent although difference of electronegativity is 1.9)
(iii) Metallic and Nonmetallic Nature : Generally values of electronegativity of metallic elements are low, whereas electronegativity values of nonnmetals are high.
(iv) Partial Ionic
Character in Covalent bonds Partial ionic characters are generated in covalent compounds by the difference of electronegativities.
Hanny and smith calculated percentage of ionic character from the difference of electronegativity.
Percentage of ionic character =


XA is electronegativity of element A
XB is electronegativity of element B
Δ = XA – XB
(v) Bond length
When difference of electronegativities of atoms present in a molecule is increased, then bond length decreases. Shoemaker and stephensen determined.

(vi) Bond Strength & Stability
Bond strength and stability of A – B increases on increase in difference of electronegativities of atoms A and B bonded A – B. Therefore H – F > H – Cl > H – Br > H – I
Ex.10 Electronegativity of which of the following is high ?
(1) –CH3(sp3)
(2) H2C = CH2(sp2)
(3) CH ≡ CH(sp)
(4) Equal in all
Ans. (3)
Ex.11 CF3NH2 is not a base, whereas CH3NH2 is a base. What is the reason ?
Sol. Due to high electronegativity of F tendency of donating the lone pair of electrons present on N will be less
Ex.12 OF2 is called oxygen difluoride, whereas Cl2O is called dichlorine monoxide. Why ?
Sol. Electronegativity of O in OF2 is less than F. Therefore, there will be positive charge on oxygen and negative charge on fluorine. Whereas in Cl and O, electronegativity of Cl is less than that of O therefore there will be positive charge on Cl and negative charge on O. Positive charge is written first followed by negative charge.
Ex.13 Calculate the electronegativity of fluorine from the following data :
EH – H = 104.2 kcal mol–1,
EF–F = 36.6 kcal mol–1
EH–F = 134.6 kcal mol–1,
XH = 2.1
Sol. Let the electronegativity of fluorine be XF.
Applying Pauling's equation.

In this equation, dissociation energies are taken in kcal mol–1.

Ex.14 The electron affinity of chlorine is 3.7 eV. How much energy in kcal is released when 2 g of chlorine is completely converted to Cl– ion in a gaseous state ? (1 eV = 23.06 kcal mol–1)
Sol.
35.5 3.7 × 23.06 kcal
∴ Energy released for conversion of 2 g gaseous chlorine into Cl– ions
 × 2 = 4.8 kcal
× 2 = 4.8 kcal
Ex.15 Calculate the electronegativity of fluorine from following data :
EH–H = 104.2 kcal mol–1
EF–F = 36.6 kcal mol–1
EH–F = 134.6 kcal mol–1
Electronegativity of H is 2.05.
Sol. On Paulling scale :

(using B.E. in kcal mol–1)

From (i)
 = = 1.5534
= = 1.5534
xF = xH + 1.4434 = 2.05 + 1.5534 = 3.6034
METALLIC PROPERTY
Metals have the tendency to form cations by loss of electrons and this property makes the elements as electropositive elements or metals.




OXIDES
Oxygen react with all elements except noble gases, Au, Pd and Pt to form oxides. In general, metallic oxides (O2–), peroxides  and super oxides
and super oxides  are ionic solids.
are ionic solids.
The tendency of group IA metals (alkali metals) to form oxygen rich compounds increases from top to bottom i.e. with increasing cation radii and decreasing charge density on the metal ion.
IIA metals also show the similar trend. Except Be, the IIA metals react with oxygen at normal conditions to form normal ionic oxides and at high pressure of O2, they form peroxides (CaO2, SrO2, BaO2). Oxides of metals are called as basic anhydries as most of them combine with water forming hydroxides with no change in oxidation state of metals.
Oxides of IA and IIA dissolve in water forming basic solution where as other oxides do not dissolve in water.

Oxygen combines with many non-metals to form covalent oxides such as CO, CO2, SO2, P4O10, Cl2O7 etc.
Non-metals with limited supply of oxygen usually form oxides in which non-metals are present in lower oxidation states where as with excess of oxygen, oxides with higher oxidatin state are formed. Oxides of non-metals are called as acid anhydrides as most of them dissolve in water forming acids of oxy-acids.
P4O10 + 6H2O → 4H3 PO4 ;
SO3 + H2O → H2SO4 : Cl2O7 + H2O → 2HClO4
- In a group, basic nature of oxides increases or acidic nature decreases. Oxides of the metals are generally basic and oxides of the non-metals are acidic. The oxides of the matalloids are amphotetric in nature. The oxides of Al, Zn, Sn, As and Sb are amphoteric.
- In a period the nature of the oxides varies from basic to acidic.
Na2O | MgO | Al2O3 | SiO2 | P4O10 | SO3 | Cl2O7 |
Strongly | basic | Basic | amphoteric | Weakly Acidic | acidic | Acidic |
Strongly acidic CO, N2O, NO and H2O are neutral oxides.
Periodic Trends in Chemical Properties of Elements
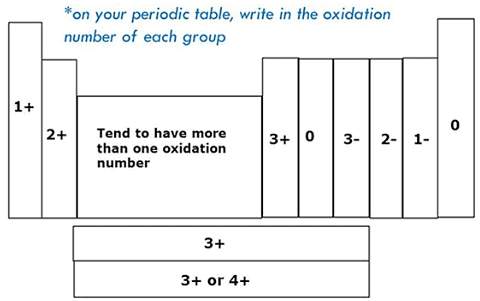
Oxidation State Or Valency
- Oxidation State or valency or valence is one of the most fundamental properties of the elements and it can be studied with the help of electronic configuration or by the number of electrons constituting the valence shell (outermost shell) of an atom.
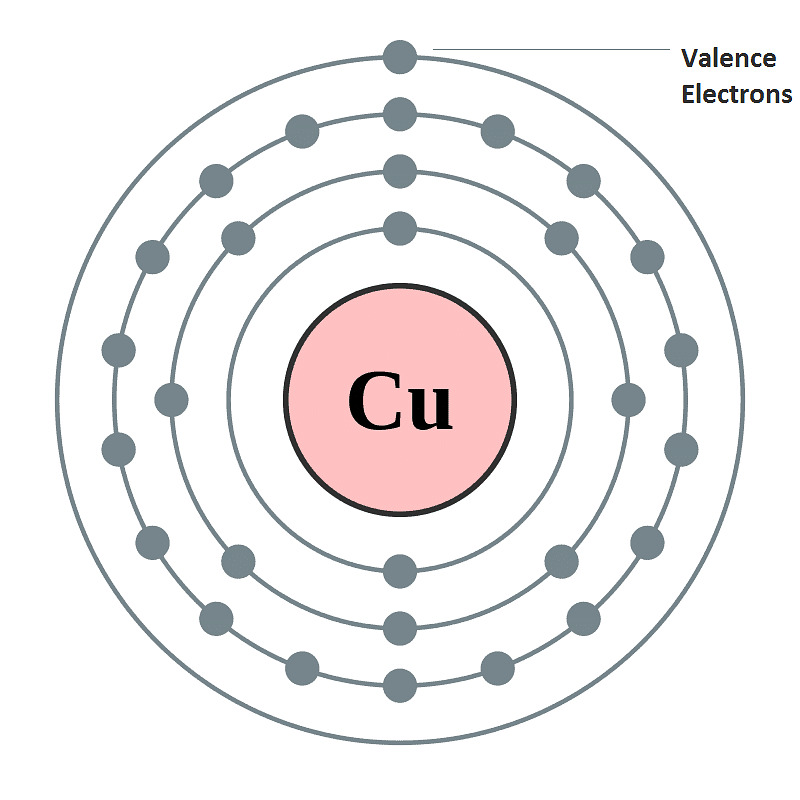
- Electrons which are found in the outermost shell are generally known as valence electrons and the number of valence electrons determines the valency or valence of an atom.
- The valency of atoms of s-block and p-block elements are generally given as the number of valence electron or eight minus the number of valence electrons.
- Whereas, in d-block and f-block elements valency is determined not only on the basis of valence electrons but also on d and f orbital electrons. However, the general valencies of these d and f block elements are 2 and 3.

Variation Of Oxidation State within a Period
While moving left to right across a period, the number of valence electrons of elements increases and varies between 1 to 8. But the valency of elements, when combined with H or O first, increases from 1 to 4 and then it reduces to zero. Consider two compounds containing oxygen Na2O and F2O. In F2O, the electronegativity of F is more than oxygen. Hence, each of F atoms will attract one electron from oxygen i.e. F will show -1 oxidation state and O will show +2 oxidation state. Whereas, in the case of Na2O, oxygen is highly electronegative than sodium atom. So oxygen will attract two electrons from each sodium atom showing -2 oxidation state and Na will have +1 oxidation state. The oxidation state of the element represents the charge possessed by an atom due to loss or gain of electrons (due to the electronegativity difference between the combining atoms) in the molecule.Variation Of Oxidation State within a group:
As we move down in a group the number of the valence electron does not change. Hence, all the elements of one group have the same valency.
Guidelines For Assigning Oxidation States:
• Oxidation states of elements like O2, S8, H2, P4, Fe etc is zero.
• Oxygen has an oxidation state of -2. But in its peroxides like Na2O2 and H2O2 , it has -1 as its oxidation state
• Similarly, hydrogen has +1. But in Metal Hydrides, such as, NaH, LiH etc, it has -1
• Some elements have the same oxidation states as in their compounds such as
• Halogens have -1 except the time they form a compound with one another or Oxygen.
• Alkali Metals such as Na, K, Rb, -Li, Cs; have +1
• And Alkali Earth Metals have +2 such as Mg, Ca, Ba, -Be, Sr etc
Anomalous Periodic Properties of Second Period Elements
All elements are special in their own way even if there are certain trends that have been observed by scientists over the years. The elements belonging to the second period display periodic properties that are especially anomalous. Let’s take a look at the elements that belong to the second period first:
It has been observed that Lithium, Beryllium, Boron, Carbon, Nitrogen, Oxygen, and Fluorine have slightly different periodic properties than the rest of the elements belonging to Groups 1, 2, 13-17 respectively. For example, Lithium and Beryllium form covalent compounds, whereas the rest of the members of Groups 1 and 2 form ionic compounds. Also, the oxide that is formed by Beryllium when it reacts with Oxygen is amphoteric in nature, unlike other Group 2 elements that form basic oxides. Yet another example is that of Carbon which can form stable multiple bonds, whereas Si=Si double bonds are not very common.
So, it has clearly been established that the second-period elements are different. In fact, they display periodic properties that are similar to the second element of the next group (i.e. Lithium is similar to Magnesium and Beryllium to Aluminium) or in other words, they have a diagonal relationship.
The reasons for differences in periodic properties and hence in chemical behavior are:
• Small size of these atoms
• High electronegativity
• Large charge/radius ratio
• These elements also have only 4 valence orbitals available (2s and 2p) for bonding as compared to the 9 available (3s, 3p, and 3d) to the other members of the respective groups, so their maximum covalency is 4. (This is why Boron can only form [BF4]– whereas Aluminium can form [AlF6]3-).
|
127 videos|244 docs|87 tests
|
FAQs on Periodic Trends in Properties of Elements - Chemistry Class 11 - NEET
| 1. What is the effective nuclear charge and how does it impact the physical properties of elements? |  |
| 2. How does the atomic radius change as you move across a period in the periodic table? |  |
| 3. What is the trend in ionization energy as you move down a group in the periodic table? |  |
| 4. How does electronegativity vary across a period in the periodic table? |  |
| 5. Why do elements within a group in the periodic table have similar chemical properties? |  |

|
Explore Courses for NEET exam
|

|