Detailed Overview: Environmental Chemistry | Science & Technology for UPSC CSE PDF Download
| Table of contents |

|
| Environment |

|
| Environmental Pollution |

|
| 1. Air Pollution |

|
| 2. Water Pollution |

|
| 3. Soil or Land Pollution |

|
| 4. Radioactive Pollution |

|
| Pollutants |

|
Environment
Environmental chemistry is the branch of chemistry which is concerned with the chemical phenomenon occurring in the environment.
Classification of Environment
1.Atmosphere
Atmosphere is a gaseous mixture of air that surrounds the earth.
Its different layers are as follows:
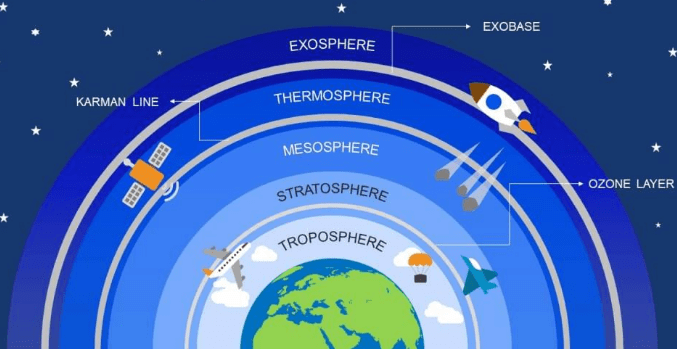 Layers of Atmosphere
Layers of Atmosphere
(i) Troposphere: It is the lowest region of the atmosphere extending from earth’s surface to the lower boundary of the stratosphere. It contains water vapour and is greatly affected by air pollution.
(ii) Stratosphere: The layer of the earth’s atmosphere above the troposphere and below the mesosphere, is called stratosphere. Ozone layer is too present in this region.
(iii) Mesosphere: It is the region of the earth’s atmosphere above the stratosphere and below the Thermosphere. It is the coldest region (temperature – 2 to 92°C) of atmosphere.
(iv) Thermosphere: The upper region of the atmosphere above the mesosphere is called Thermosphere. It is the hottest region (temperature up to 1200°C).
(v) Exosphere: It is the uppermost region of atmosphere. It contains atomic and ionic O2, H2 and He.
2. Hydrosphere
It is the aqueous envelop of the earth e.g., oceans. lakes etc.
 Hydrosphere
Hydrosphere
3. Lithosphere
The solid rocky portion of the earth constitute the lithosphere.
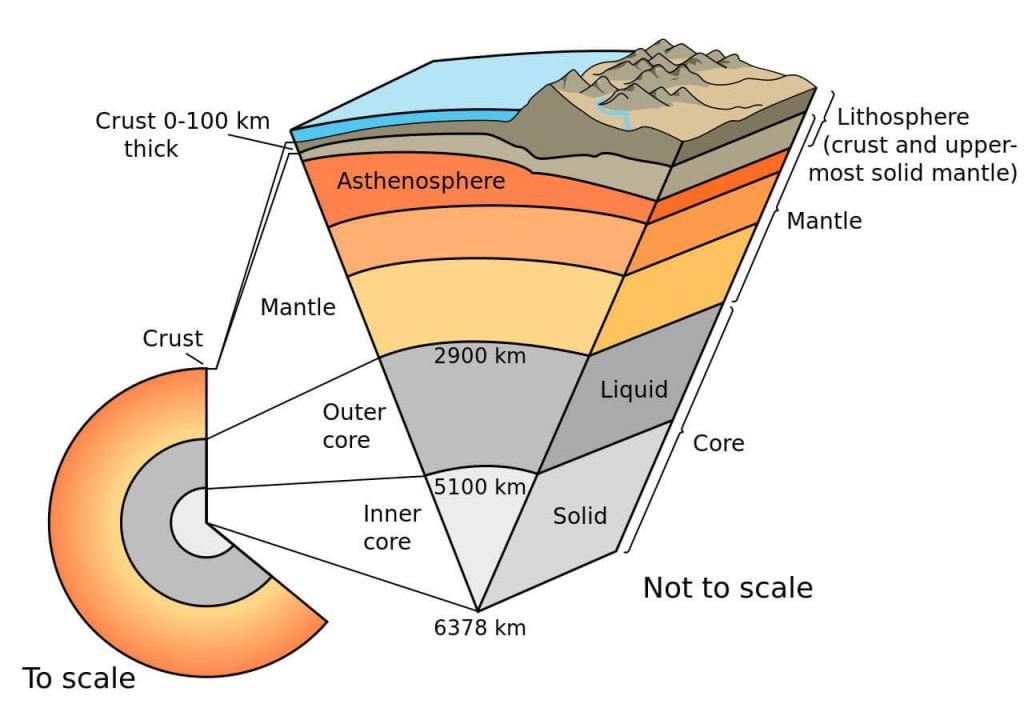 Lithosphere
Lithosphere
4. Biosphere
The biological envelop which supports the life is called biosphere. e.g., animal, human beings.
 Biosphere
Biosphere
Environmental Pollution
It may be described as contamination of environment with harmful wastes mainly arising from certain human activities. These activities release materials which pollute atmosphere, water and soil.
Types of Pollution
(i) Natural pollution
This type of pollution is caused by the natural sources e.g., volcanic eruptions, release of methane by paddy fields and cattles, forest fires etc.
 Natural Pollution
Natural Pollution
(ii) Man-made pollution
This type of pollution is resulting from human activities like burning of the fuels, deforestation, industrial effluents, pesticides etc.
1. Air Pollution
Air pollution occurs when the concentration of a normal component of the air or a new chemical substance added or formed in air, build up to undesirable proportions causing harm to humans, animals, vegetation and materials. The chemical substances and particles causing pollution are called air pollutants.
 Air Pollutants
Air Pollutants
The major air pollutants are:
(i) Carbon monoxide(CO): It is produced by incomplete combustion of gasoline in motor vehicles, wood. coal, incineration and forest fires. It induces headache, visual difficulty, coma or death.
It blocks the normal transport of oxygen from the lungs to other parts of the body, by combining with haemoglobin of the blood. (Its affinity towards haemoglobin is about 200 times more than the oxygen.)
(ii) Sulphur dioxide(SO2): It is produced by petrol combustion, coal combustion, petrol refining and smelting operation.
It obstructs the movement of air in and out of lungs. It is particularly poisonous to trees causing chlorosis and dwarfing. In the presence of air, it is oxidised to SO3 which is also an irritant.
2SO2 + O2 (air) → 2SO3
Taj Mahal is reported to be affected by SO2 and other pollutants released by oil refinery of Mathura.
(iii) Oxides of nitrogen: NO2 and NO are obtained by combustion of coal, gasoline. natural gas, petroleum refining, chemical industries and tobacco smoke. In upper atmosphere, these are emitted by high flying jets and rockets.
Breathing NO2 causes chlorosis to plants and chronic lung conditions leading to death in human beings . These oxides destroy ozone layer.
(iv) Smoke, dust: These are obtained in cement works, iron and steel works, gas works and power generating stations. Coal miners suffer from black lung disease and textile workers suffer from white lung disease.
 Smoke and Dust
Smoke and Dust
(v) Ammonia: It is produced by fertilizer works.
(vi) Mercaptans: These are obtained from oil refineries. coke ovens etc.
(vii) Zn and Cd: These are obtained from zinc industries.
(viii) Freon (or CCl2F2): Their source is refrigerator.
Smog
It is a mixture of smoke (composed of tiny particles of carbon, ash and oil etc from coal combustion) and fog in suspended droplet form.
It is of two types:
1. London smog or classical smog
It is coal smoke plus fog. The fog part is mainly SO2 and SO3. It has sulphuric acid aerosol. It causes bronchial irritation and acid rain. It is reducing in nature and occurs in cool humid climate.
2. Photochemical smog or Los Angeles smog
The oxidized hydrocarbons and ozone in a warm, dry and sunny climate cause photochemical smog. Its brown colour is due to the presence of NO2.
The nitrogen dioxide by absorbing sunlight in blue and UV region decomposes into nitric oxide and atomic oxygen followed by a series of the other reactions producing O3, formaldehyde, acrolein and peroxyacetyl nitrates.
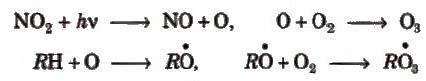
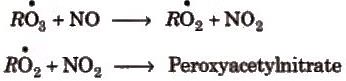
Hydrocarbons + O2, NO2 NO, O, O3 Peroxides, formaldehyde, peroxyacetyl nitrate (PAN), acrolein etc.
It is oxidising in nature and causes irritation to eyes, lungs, nose, asthmatic attack and damage to plants.
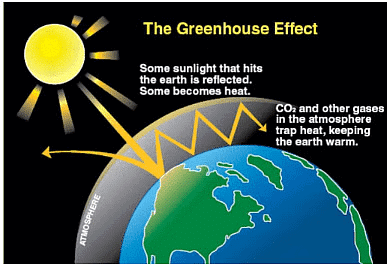
Green House Effect and Global Warming
The phenomenon in which atmosphere of earth traps the heat coming from the sun and prevents it from escaping into the outer space is called green house effect. Certain gases, called green house gases [carbon dioxide, methane, ozone, chlorofluorocarbon compounds (CFCs) and water vapour] present in the atmosphere absorb the heat given by earth and radiate it back to the surface of the earth. Thus, warming of the earth leads to the warming of air due to green house gases, which is called global warming.
 Green House Effect and Global Warming
Green House Effect and Global Warming
Consequences of Green House Effect (or Global Warming)
1. The green house gases are useful in keeping the earth warm with an average temperature of about 15° to 20°C.
2. There may be less rainfall in this temperature zone and more rainfall in the dried areas of the world.
3. Increase in the concentration of CO2 in the atmosphere leads to increase in the temperature of the earth’s surface. As a result evaporation of surface water will increase which further helps in the rise of temperature and results in the melting of glaciers and polar ice caps and hence, level of sea water may rise.
Acid Rain
The pH of normal rain water is 5.6 due to the dissolution of CO2 from atmosphere.
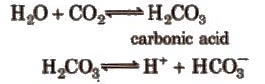
When the pH of rain water drops below 5 ppm, it is called acid rain (by Robert Augus.) Oxides of N ans S are responsible for making rain water acidic. Much of the NOx and SOx entering in the atmosphere are converted into HNO3 and H2SO4 respectively. The detailed photochemical reactions occurring in the atmosphere are given as:

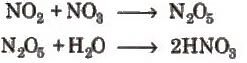
HNO3 is removed as a precipitate or as particulate nitrates after reaction with bases (like NH3, particulate lime etc).

The presence of hydrocarbons and NOx step up the oxidation rate of the reaction. Soot particles are also known to be strongly involved in catalysing the oxidation of SO2.
Acid rain causes extensive damage to building and sculptural materials of marble, limestone, slate. mortal’ etc.

Stratospheric Pollution (Depletion of Ozone Layer)
Ozone is a light bluish gas and absorbs UV radiations of the sun which are harmful to living beings. But nowadays ozone layer is being depleted by CFCs (chlorofluorocarbons).
UV radiations cause the chlorofluorocarbons to dissociate to fOl1D highly reactive chlorine free radical which reacts with ozone to form chlorine monoxide.


CI* (free radical) can react With more O3.
Ozone hole is formed over Antarctica and some parts of non – polar regions also.
In other parts of stratosphere NO2, CH4 react with ClO* and Cl* respectively and act as natural sink for ClO* and Cl*.
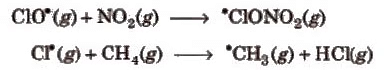
These reactions consume Cl* and ClO* hindrance to ozone depletion.
[In Antarctica, during winters, special types of clouds, called polar stratospheric clouds (PSCs)are formed.
These clouds are of two types:
Type I Clouds: They contain some solidified nitric acid trihydrate (HNO3 * 3H2O) formed at about -77°C.
Type II Clouds: They contain some ice formed at about – 85°C. These clouds play important role in ozone depletion by hydrolysing chlorine nitrate.

Hypochlorous acid and Cl2 are formed which are reconverted into reactive chlorine atoms with the help of sunlight which causes ozone depletion.
Polar vortex: During winters, when polar stratospheric clouds are formed over Antarctica. Stable wind patterns in the stratosphere encircle the continent which is called polar vortex. It is tight whirlpool of winds which is so rigid that air within it is isolated from the sun and forms the warmer air of temperate region to fill up ozone hole.
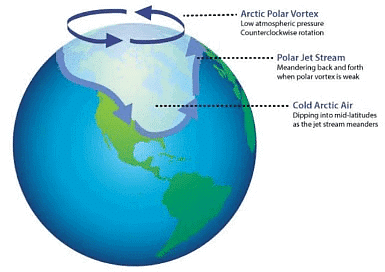 Polar Vortex
Polar Vortex
Consequences of Depletion of Ozone Layer
(a) Loss of sight-The UV radiation damage the cornea and lens of the eyes.
(b) Effect on immune system-The UV radiations are also likely to suppress immune system.
(c) Skin cancer-This type of radiation is known to be cancer-causing agent.
2. Water Pollution
The contamination of water by foreign substances which would cause health hazards and make it unfit for all purposes (domestic, industrial or agriculture etc) is known as water pollution. The polluted water may have foul odour, bad taste, unpleasant colour etc.
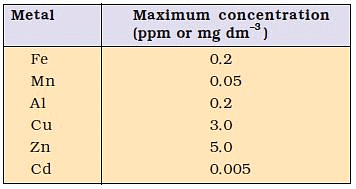
Maximum prescribed concentration of some metals in drinking water is as:
Sources of Water Pollution
(i) Domestic sewage: Discharge from kitchens, baths, etc.
(ii) Industrial water: Wastes from manufacturing processes which includes acids, alkalies, pesticides, insecticides, metals. fungicides etc.
(iii) Oil: From oil spills or washings of automobiles.
(iv) Atomic explosion: Processing of radioactive materials.
(v) Suspended particles (organic or inorganic): Viruses, bacteria, algae, protozoa etc.
(vi) Wastes from fertilizers: Industries such as phosphates, nitrates, ammonia etc.
(vii) Clay: Ores, minerals, fine particles of soil.
Effects of Impurities in Water
(a) Fluorides: Mottling of teeth enamel, above 1 mg/L fluoride causes fluorosis.
(b) Sulphates: Sulphates of Na, K, Mg cause diarrhoea.
(c) Lead: It damages kidney, liver, brain and central nervous system.
(d) Cadmium and mercury: They cause kidney damage.
(e) Zn: It causes dizziness and diarrhoea. .
(f) Arsenic: It can cause cramps and paralysis.
(g) Phosphates from fertilizers: They promote algae growth and reduce dissolved oxygen concentration of water. This process is known as eutrophication.
Aerobic and Anaerobic Oxidation
The oxidation of organic compounds present in sewage in the presence of good amount of dissolved or free oxygen (approx, 8.5 mlJL) by aerobic bacteria is called aerobic oxidation. When dissolved or free oxygen is below a certain value, the sewage is called stale.
Anaerobic bacteria bring out putrefaction by producing H2S, NH3, CH4, (NH4)2S etc. This type of oxidation is called anaerobic oxidation.
The optimum value of dissolved oxygen for good quality of water is 4·6 ppm (4-6 mg/L). The lower the concentration of dissolved oxygen, the more polluted is the water.
- Biological Oxygen Demand (BOD): It is defined as the amount of free oxygen required for biological oxidation of the organic matter under aerobic conditions at 20°C for a period of five days. Its unit is mg/L or ppm. An average sewage has BOD of 100 to 150 mg/L.
- Chemical oxygen demand (COD): It is the measure of all types of oxidisable impurities (biologically oxidisable and biologically inert organic matter such as cellulose) present in the sewage. COD values are higher than BOD values.
Control of Water Pollution
(i) Recycling of waste water
(ii) Use of chemicals: Lead poisoning can be cured by giving the patient an aqueous solution of calcium complex of EDTA. Lead ions displace calcium in the EDTA complex to form chelated lead and Ca2+. The soluble lead chelate is excreted with the urine.
Ca – EDTA + Pb2+ → Pb – EDTA + Ca2+
(iii) Special techniques such as adsorption, ion exchangers, reverse osmosis, electrodialysis etc.
(iv) Waste water reclamation
Sewage
Sewage, also known as wastewater, is water that has been used and contaminated by human activities. It originates from various sources, including households, industries, and businesses. Sewage typically contains a mixture of:
- Human Waste: Feces and urine from toilets.
- Water from Domestic Activities: Used water from sinks, showers, washing machines, and dishwashers.
- Industrial Waste: Effluents from factories and industrial processes.
- Stormwater: Rainwater that runs off roofs, roads, and other surfaces, often carrying pollutants like oil, chemicals, and debris.
Sewage can be divided into two main categories:
- Domestic Sewage: Wastewater from homes and residential areas.
- Industrial Sewage: Wastewater from industrial activities, which may contain hazardous chemicals and require special treatment.
Composition of Sewage
Sewage contains a variety of substances, including:
- Organic Matter: Such as food waste, human waste, and other biodegradable materials.
- Inorganic Matter: Such as sand, grit, and dissolved minerals.
- Pathogens: Bacteria, viruses, and parasites that can cause diseases.
- Nutrients: Such as nitrogen and phosphorus, which can lead to water pollution and eutrophication.
- Chemicals: Including household cleaning agents, pharmaceuticals, and industrial chemicals
 |
Download the notes
Detailed Overview: Environmental Chemistry
|
Download as PDF |
Sewage Treatment
It involves the following steps:
(i) Preliminary process: Passing sewage through screens to remove large suspended matter and then through mesh screens to remove solids, gravels, silt etc.
(ii) Settling process (sedimentation): The residual water when allowed to stand in tanks, the oils and grease, float on the surface and skimmed off and solids settle down. The colloidal material is removed by adding alum, ferrous sulphate etc. Primary sludge can be separated.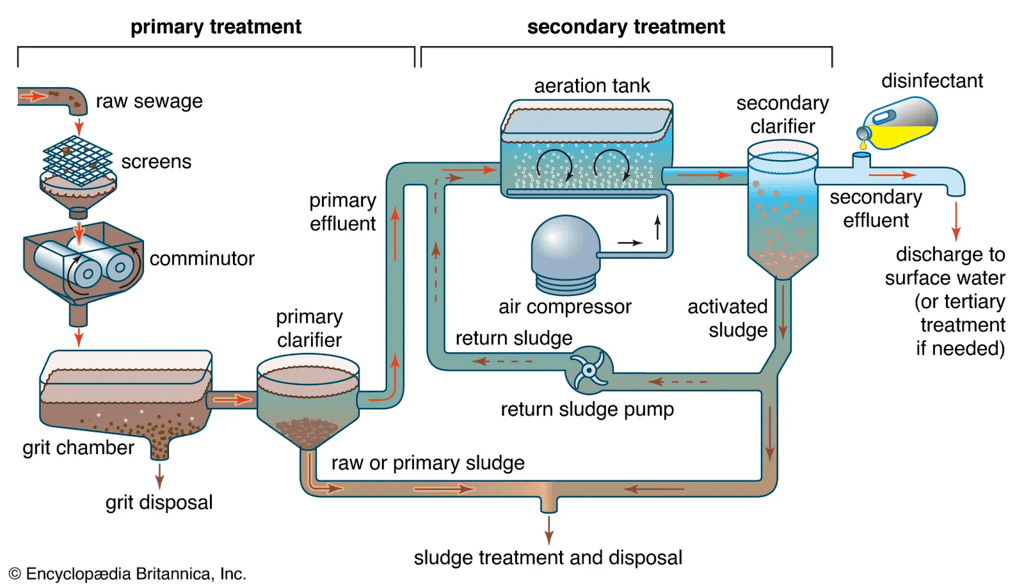
(iii) Secondary treatment or biological treatment: It is aerobic chemical oxidation or aeration which converts carbon of the organic matter to CO2, nitrogen into NO and finally into nitrite and nitrates. Dissolved bases form salts such as NH4O2, NH4NO3 and Ca(NO3)2etc., and secondary sludge is obtained.
(iv) Tertiary treatment: It is treatment of waste water with time for removal of phosphate which is then coagulated by adding alum and ferric chloride and removed by filtration. Water is disinfected by adding chlorine. Secondary sludge forms a good fertilizer for soil as it contains nitrogen and phosphorus compounds.
3. Soil or Land Pollution
The addition of substances in an indefinite proportion changes the productivity of the soil. This is known as soil or land pollution.

Sources of Soil Pollution
(i) Agricultural pollutants e.g., chemicals like pesticides, fertilizers, bactericides, fumigants. insecticides, herbicides, fungicides.
(ii) Domestic refuge and industrial wastes.
(iii) Radioactive wastes from research centres, and hospitals.
(iv) Soil conditioners containing toxic metals like Hg, Pb, As. Cd etc.
(v) Farm wastes from poultry, dairies and piggery farms.
Control of Soil Pollution
(i) Use of manures- Manures prepared from animal dung is much better than the commonly used fertilizers.
(ii) Use of bio- fertilizers- These are the organisms which are inoculated in order to bring about nutrient enrichment of the soil. e.g., nitrogen fixing bacteria and blue-green algae.
(iii) Proper sewerage system- A proper sewerage system must be employed and sewage recycling plants must be installed.
(iv) Salvage and recycling- Rag pickers remove a large number of waste articles such as paper, polythene, card board, rags, empty bottles and metallic articles. These are subjected to recycling and this helps in checking soil pollution.
4. Radioactive Pollution
Cosmic rays that reach the earth from outer space and terrestrial radiation from radioactive elements are natural radiations. This natural or background radiation is not a health hazard due to its low concentration.
Man-made sources of radiations include mining; and refining of plutonium and thorium, atomic reactors and nuclear fuel. These are produced during preparation of radio-isotopes. These are of two types: electromagnetic (radio waves UV, IR, α-rays) and particulate.
Other Sources of Radioactive Pollution
(i) Atomic explosions: Atomic explosions produce radioactive particles which are thrown high up into the air as huge clouds.
The process releases large amount of energy as heat due to atomic -explosion nuclear fallout. These radioactive elements may reach the human beings through food chain.
(ii) Radioactive wastes Wastes from atomic power plants come in the form of spent fuels of uranium, and plutonium. People working in such power plants, nuclear reactors, fuel processors etc., are vulnerable to their exposure.
(iii) Radio isotopes Many radioactive isotopes like C14, I125, P32 and their compounds are used in scientific researches. The waste water of these research centres contain the radioactive elements which may reach the human beings through water and food chains.
Effects of Radiations
- Strontium-90 accumulates in the bones to cause bone cancer and tissue degeneration in number of organs.
- 1-131 damages WBCs, bone marrow, lymph nodes and causes skin cancer, sterility and defective eye sight.
- These may cause ionisation of various body fluids, chromosomal aberrations and gene mutations.
- Radioactive iodine may also cause cancer of thyroid glands.
- Cesium-137 brings about nervous, muscular and genetic change.
- Uranium causes skin cancers and tumours in the miners.
- Radon-222 causes leukemia, brain tumours and kidney cancers.
Bhopal Gas Tragedy
On Dec. 2, 1984 a dense cloud of methyl isocyanate gas (Mlq leaked from a storage tank of the Union Carbide ltd plant in Bhopal. It caused a great loss of life to people and animals. Methyl Isocyanate was prepared by the reaction of methyl amine with phosgene and stored in abundance.

Pollutants
Any substance produced either by a natural source or by human activity which causes adverse effect on the environment is called pollutant.
Pollutants can be of the following types depending upon the following factors:
Classification on the Basis of Their Degradation
(i) Biodegradable pollutants
Pollutants capable of being degraded by biological or microbial actions are called biodegradable pollutants. E.g., domestic sewage.

(ii) Non-biodegradable pollutants
The substances which are normally not acted upon by microbes are called non-biodegradable pollutants. These undergo biological magnification.
They can further be of two types as below:
- Wastes, e.g., glass, plastic, phenols.
- Poisons, e.g., radioactive substances, Hg salts, pesticides, heavy metals.
Classification on the Basis of Their Occurrence in Nature
(i) Primary pollutants: These are present in same form in which these are added by man e.g., DDT, pesticides, fertilizers etc.
(ii) Secondary pollutants: These occur in different forms and are formed by the reaction between the primary pollutants in the presence of sunlight e.g., HNO3, H2SO4 PAN, ozone etc.
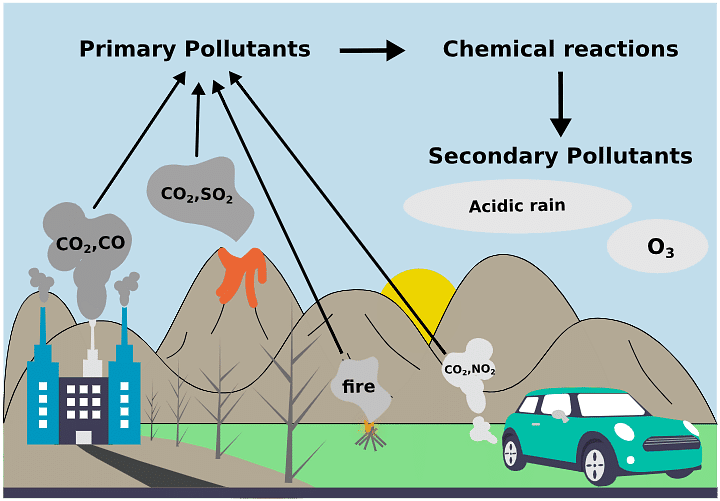
Classification on the Basis of Their Existence in Nature
(i) Quantitative pollutants: These are naturally present in nature and also added by man. These become pollutants when their concentration reaches beyond a threshold value in the environment, e.g., CO2, nitrogen oxide etc.
(ii) Qualitative pollutants: These are not present in the nature but are added by nature only due to human activities. e.g., pesticides. fungicides. herbicides etc.
Tropospheric Pollution: It is caused by gaseous pollutants and particulate matter.
Gaseous air pollutants: Oxides of sulphur (SOx), oxides of nitrogen (NOx), oxides of carbon (CO, CO2), hydrogen sulphide (H2S), hydrocarbons etc.
Particulate pollutants: Dust, fumes, mist, smoke etc.
Green Chemistry - An Alternative Tool for Reducing Pollution
Green chemistry may be called chemistry involved in the design, development, and implementation of chemical products and processes to reduce or eliminate the use and generation of substances hazardous to human health and the environment.

Thus, the goal of green chemistry is ‘to promote the development of products and processes that reduce or eliminate the use or generation of toxic substances associated with the design, manufacture, and use of hazardous chemicals. Some important principles and method of green chemistry are-
- It is better to prevent waste than to treat or clean up waste after it is formed.
- Synthetic methods should be designed to maximize the incorporation of all materials used in the process into the final product.
- Whenever possible, synthetic methodologies should be designed to use and generate substances that possess little or no toxicity to human health and the environment.
- Chemical products should be designed to preserve efficiency of function while reducing toxicity.
- The use of auxiliary substance (e.g., solvents, separation agents etc.) should be avoided as far as possible.
- Energy requirements should be recognized for their environmental and economic impacts and should be minimized.
- Synthetic methods should be conducted at ambient temperature and pressure.
|
114 videos|429 docs|209 tests
|
FAQs on Detailed Overview: Environmental Chemistry - Science & Technology for UPSC CSE
| 1. What are the main sources of air pollution? |  |
| 2. How does water pollution affect marine life? |  |
| 3. What are the effects of soil pollution on agriculture? |  |
| 4. How does radioactive pollution occur and what are its consequences? |  |
| 5. How does ozone layer depletion contribute to environmental pollution? |  |























