Preparation, Purification & Properties of Colloidal Solutions | Chemistry Class 12 - NEET PDF Download
Preparation of Colloids
Stable colloids are also known as lyophilic sols, in these strong forces of interaction exist between the dispersed phase and the dispersion medium. These are prepared by the following suitable methods.
 Colloidal Dispersions
Colloidal Dispersions
Thus, there are two ways by which the lyophobic sols can be prepared:
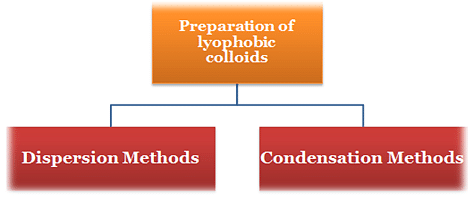
(i) Condensation methods: By splitting coarse aggregates of a substance into colloidal size.
(ii) Dispersion methods: By aggregating very small particles (atoms, ions or molecules) into colloidal size.

1.Condensation Methods
(i) By Exchange of Solvents
- If a solution of sulphur or phosphorus prepared in alcohol is poured into water, a colloidal solution of sulphur or phosphorus is obtained due to low solubility of sulphur or phosphorus is obtained due to low solubility in water.
- Thus, there are a number of substances whose colloidal solutions can be prepared by taking a solution of the substance in one solvent and pouring it into another solvent in which the substance is relatively less soluble.
(ii) By change of physical state
Colloidal solutions of certain elements such as mercury and sulphur are obtained by passing their vapour through cold water containing a stabilizer (an ammonium salt or citrate).
2. Chemical methods
The chemical methods involve chemical reactions in a medium in which the dispersed phase is sparingly soluble. A condition of supersaturation is produced but the actual precipitation is avoided.
Some familiar reactions used are:
(i) Double decomposition
(i) Arsenious Sulphide sol: A 1% solution of arsenious oxide is prepared is hot water. The solution is cooled, filtered and is then gradually in hot water saturated with hydrogen sulphide. This is continued till an intense yellow-coloured solution is obtained. Excess of H2S is removed by bubbling hydrogen through the solution.
As2O3 + 3H2S  As2S3 + 3H2O
As2S3 + 3H2O
Yellow sol
(ii) Antimony Sulphide sol: A 0.5% solution of potassium antimonyl tartrate is added drop by drop to water saturated with H2S, whilst H2S is being passed through the solution. Orange coloured solution of antimony sulphide is obtained.

(ii) Oxidation
A colloidal solution of sulphur is obtained by passing H2O into a solution of sulphur dioxide. 2H2S + SO2  2H2O + 3S
2H2O + 3S
Sulphur sol can also be obtained when H2S is bubbled through an oxidising agent (bromine water or nitric acid).
(iii) Reduction
Colloidal solutions of metals like gold, silver, platinum, lead, etc., can be obtained when their salts solutions are acted upon by reducing agents.2AuCl3 + 3SnCl2  3SnCl4 + 2Au
3SnCl4 + 2Au
Organic reducing agents such as formaldehyde, phenylhydrazine, tannic acid, etc., can also be used.
AgNO3 + tannic acid  Silver sol
Silver sol
AuCl3 + tannic acid  Gold sol
Gold sol
(iv) Hydrolysis
Colloidal solutions of some salts can be prepared by hydrolysis. A colloidal solution of ferric hydroxide is obtained by boiling a dilute solution of ferric chloride.FeCl3 + 3H2O 

The colloidal solution of silicic acid is also obtained by hydrolysis of a dilute solution of sodium silicate with 4N hydrochloric acid which is added drop by drop with constant stirring.
3. Dispersion Methods
(i) Mechanical Dispersion
- The solid material is first finely ground by usual methods. It is then mixed with a dispersion medium which gives a coarse suspension.
- The suspension is now introduced into the colloid mill. The simplest form of colloid mill consists of two metal discs held at a small distance apart from one another and capable of revolving at a very high speed (about 7000 revolutions per minute) in opposite directions.
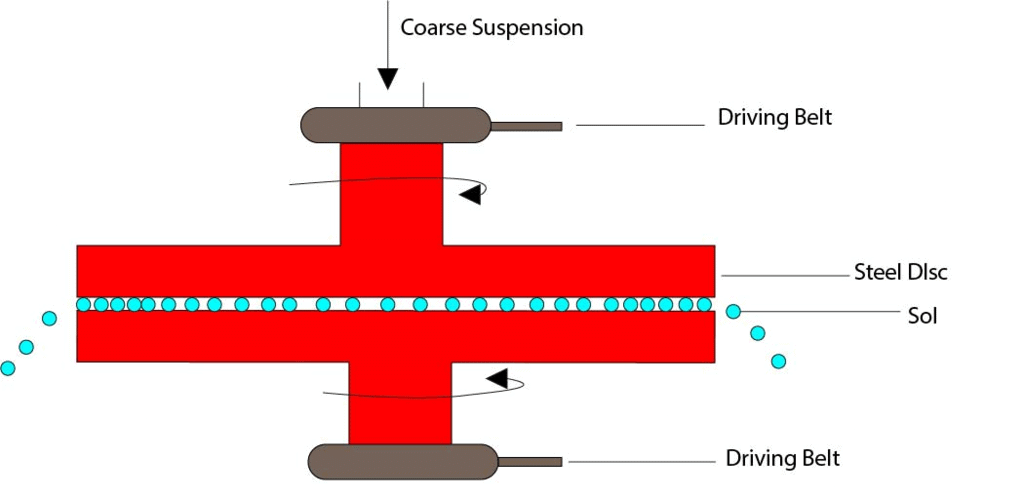 Mechanical Dispersion
Mechanical Dispersion
- The particles are ground down to colloidal size and are then dispersed in the liquid. A stabilizer is often added to stabilize the colloidal solution.
- Colloidal graphite (a lubricant) and printing ink, are made by this method. Tannin is used as a stabilizer in the preparation of colloidal graphite and gum arabic in lampblack colloidal solution (Indian ink).
(ii) Electro-Dispersion (Bredig's Arc Method)
- This method is suitable for the preparation of colloidal solutions of metals like gold, silver, platinum, etc. An arc is struck between the metal electrodes under the surface of water containing some stabilizing agents such as a track of KOH.
- The water is cooled by immersing the container in an ice bath. The intense heat of the arc vaporises some of the metal which condenses under cold water.
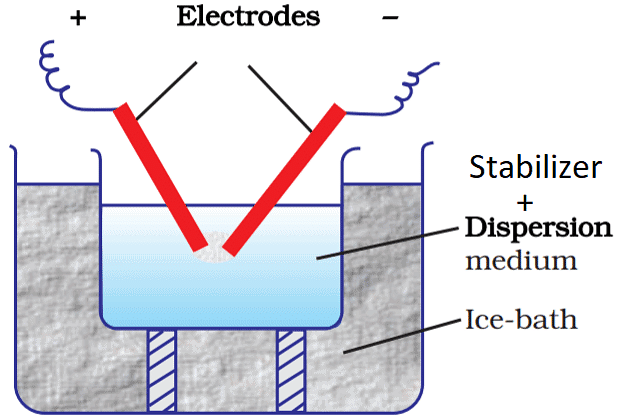 Electro - Dispersion (Bredig's Arc Method)
Electro - Dispersion (Bredig's Arc Method)
Note:
- This method is not suitable when the dispersion medium is an organic liquid as considerable charring occurs.
- This method comprises both dispersion and condensation.
(iii) Ultrasonic Dispersion
- The sound waves of high frequency are usually called ultrasonic waves. These waves can be produced when quartz crystal discs are connected with a high-frequency generator.
- The application of ultrasonic waves for the preparation of colloidal solutions was first introduced by wood and Loom is, in 1927. Various substances like oils, mercury, sulphur, sulphides and oxides of metals can be dispersed into a colloidal state very easily with the help of ultrasonic waves.
(iv) Peptization
The dispersion of a freshly precipitated material into colloidal solution by the action of an electrolyte in solution is termed peptization. The electrolyte used is called a peptizing agent.
A few examples of sols obtained by peptization are:
- Freshly prepared ferric hydroxide on treatment with a small amount of ferric chloride solution at once forms a dark reddish-brown solution. Ferric chloride acts as a peptizing agent.
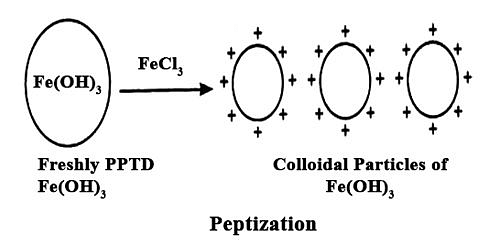
- Freshly prepared stannic oxide on treatment with a small amount of dilute hydrochloric acid forms a stable colloidal solution of stannic oxide.
- Freshly precipitated silver chloride can be converted into a colloidal solution by a small amount of hydrochloric acid.
- Cadmium sulphide can be peptized with the help of hydrogen sulphide.
The process of peptization thus involves the adsorption of suitable ions (supplied by the electrolyte added -- particularly a common ion) and electrically charged particles then split from the precipitate as colloidal particles.
Important peptizing agents: Sugar, Gum, Gelatin & Electrolytes.
Example: Freshly prepared ferric hydroxide can be converted into a colloidal state by shaking it with water containing Fe3+ or OH¯ or FeCl3
Fe(OH)3 + xFe 3+ ---------→ Fe (OH)3.xFe 3+
Precipitate Peptizing agent colloid
Purification of Colloidal Solutions
Colloidal solutions when prepared, generally contain an excessive amount of electrolytes and some other soluble impurities.
- While the presence of traces of electrolyte is essential for the stability of the colloidal solution, larger quantities coagulate it. It is, therefore, necessary to reduce the concentration of these soluble impurities to a requisite minimum.
- The process used for reducing the number of impurities to a requisite minimum is known as the purification of colloidal solution.
 Purification of Colloids
Purification of Colloids
The purification of the colloidal solution is carried out by the following methods:
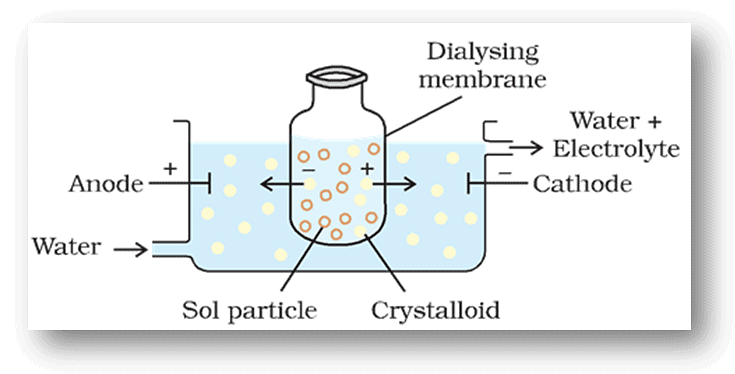
(i) Dialysis
- It is a process of removing a dissolved substance from a colloidal solution by means of diffusion through a suitable membrane.
- Since particles (ions or smaller molecules) in a true solution can pass through animal membrane (bladder) or parchment paper or cellophane sheet but not the colloidal particles, the membrane can be used for dialysis.
- The apparatus used for this purpose is called dialyser. A bag of suitable membrane containing the colloidal solution is suspended in a vessel through which fresh water is continuously flowing (Fig.)
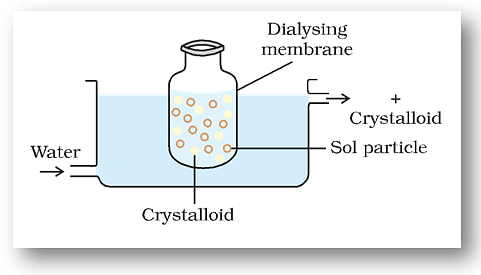 Dialysis
Dialysis
- The molecules and ions diffuse through the membrane into the outer water and the pure colloidal solution is left behind.
(ii) Electro-Dialysis
- Ordinarily, the process of dialysis is quite slow. It can be made faster by applying an electric field if the dissolved substance in the impure colloidal solution is only an electrolyte.
- The process is then named electrodialysis. The colloidal solution is placed in a bag of the suitable membrane while pure water is taken outside. Electrodes are fitted in the compartment as shown in Fig.
 Electro- Dialysis
Electro- Dialysis - The ions present in the colloidal solution migrate out to the oppositely charged electrodes.
(iii) Ultrafiltration
- Ultrafiltration is the process of separating the colloidal particles from the solvent and soluble solutes present in the colloidal solution by specially prepared filters, which are permeable to all substances except the colloidal particles.
- Colloidal particles can pass through ordinary filter paper because the pores are too large. However, the pores of filter paper can be reduced in size by impregnating with collodion solution to stop the flow of colloidal particles.
- The usual collodion is a 4% solution of nitrocellulose in a mixture of alcohol and ether. An ultra-filter paper may be prepared by soaking the filter paper in a collodion solution, hardening by formaldehyde and then finally drying it. Thus, by using ultra-filter paper, the colloidal particles are separated from the rest of the materials.
- Ultrafiltration is a slow process. To speed up the process, pressure or suction is applied. The colloidal particles left on the ultra-filter paper are then stirred with a fresh dispersion medium (solvent) to get a pure colloidal solution.
Properties of Colloidal Solutions
Various properties exhibited by the colloidal solutions are described below:
1. Colligative Properties
- Colloidal particles being bigger aggregates, the number of particles in a colloidal solution is comparatively small as compared to a true solution.
- Hence, the values of colligative properties (osmotic pressure, lowering in vapour pressure, depression in freezing point and elevation in boiling point) are of small order as compared to values shown by true solutions at the same concentrations.
2. Tyndall effect
It is a homogeneous solution placed in dark is observed in the direction of light, it appears clear and, if it is observed from a direction at right angles to the direction of the light beam, it appears perfectly dark.
- Colloidal solutions viewed in the same way may also appear reasonably clear or translucent by the transmitted light but they show a mild to strong opalescence when viewed at right angles to the passage of light, i.e., the path of the beam is illuminated by a bluish light.
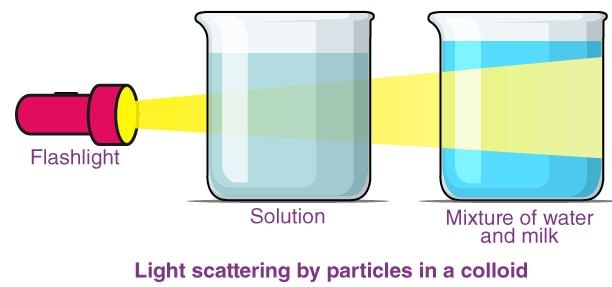
- This effect was first observed by Faraday and later studied in detail by Tyndall and is termed as the Tyndall effect. The bright cone of the light is called the Tyndall cone (Fig.).
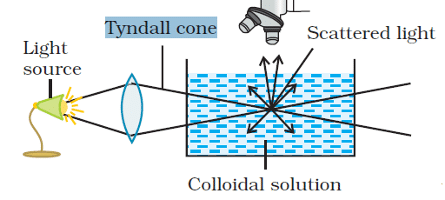
- The Tyndall effect is due to the fact that colloidal particles scatter light in all directions in space. This scattering of light illuminates the path of the beam in the colloidal dispersion.
- Tyndall effect can be observed during the projection of pictures in the cinema hall due to the scattering of light by dust and smoke particles present there.
- Tyndall effect is observed only when the following two conditions are satisfied:
(i) The diameter of the dispersed particles is not much smaller than the wavelength of the light used.
(ii) The refractive indices of the dispersed phase and the dispersion medium differ greatly in magnitude.
3. Colour
- The colour of the colloidal solution depends on the wavelength of light scattered by the dispersed particles.
- The wavelength of light further depends on the size and nature of the particles. The colour of the colloidal solution also changes with the manner in which the observer receives the light.
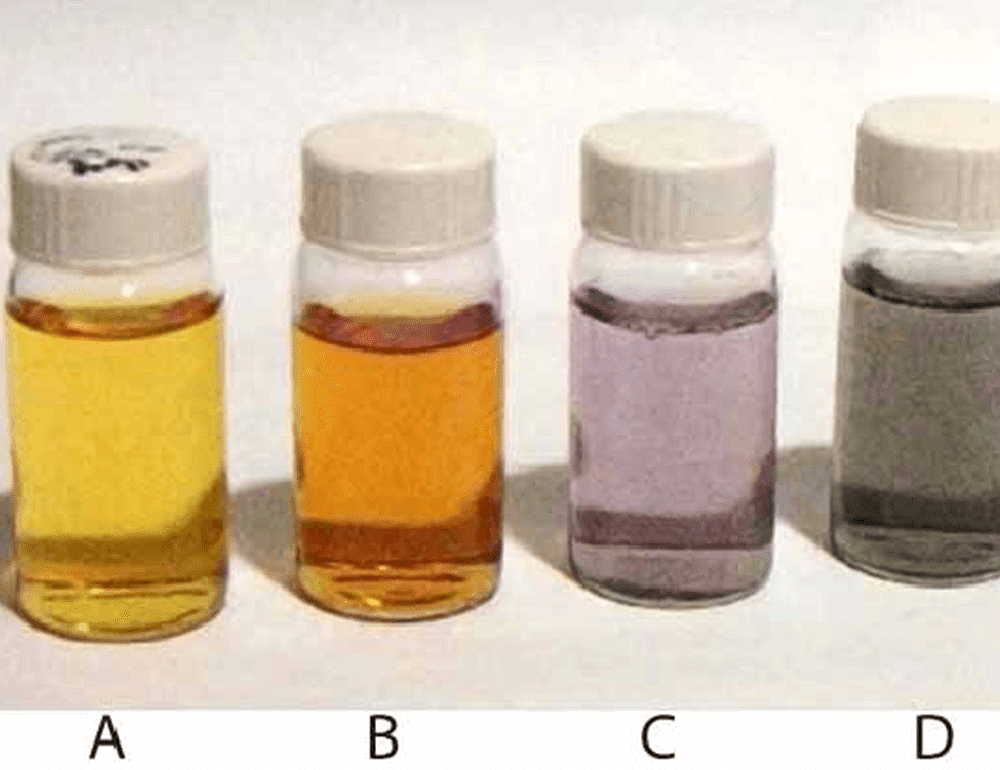
- For example, a mixture of milk and water appears blue when viewed by the reflected light and red when viewed by the transmitted light. The finest gold sol is red in colour; as the size of particles increases, it appears purple, then blue and finally golden.
4. Brownian Movement
- When colloidal solutions are viewed under a powerful ultramicroscope, the colloidal particles appear to be in a state of continuous zig-zag motion all over the field of view.
- This motion was first observed by the British botanist, Robert Brown, and is known as the Brownian movement (Fig.).
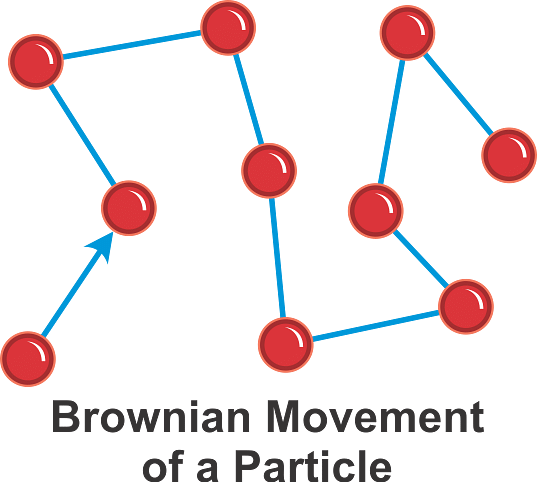
- This motion is independent of the nature of the colloid but depends on the size of the particles and the viscosity of the solution. The smaller the size and lesser the viscosity, the faster is the motion.
- The Brownian movement has been explained to be due to the unbalanced bombardment of the particles by the molecules of the dispersion medium.
- The Brownian movement has a stirring effect that does not permit the particles to settle and thus, is responsible for the stability of sols.
5. Charge on Colloidal Particles
- Colloidal particles always carry an electric charge.
- The nature of this charge is the same on all the particles in a given colloidal solution and may be either positive or negative.
- A list of some common sols with the nature of a charge on their particles is given below:

- The charge on the sol particles is due to one or more reasons, viz., due to electron capture by sol particles during electro-dispersion of metals, due to preferential adsorption of ions from solution and/or due to formulation of an electrical double layer.
- Preferential adsorption of ions is the most accepted reason. The sol particles acquire positive or negative charge by preferential adsorption of +ve or –ve ions. When two or more ions are present in the dispersion medium, preferential adsorption of the ion common to the colloidal particle usually takes place.
This can be explained by taking the following examples:
(a) When silver nitrate solution is added to potassium iodide solution, the precipitated silver iodide adsorbs iodide ions from the dispersion medium and negatively charged colloidal solution results. However, when KI solution is added to AgNO3 solution, positively charged sol results due to adsorption of Ag+ ions from dispersion medium.
(b) If FeCl3 is added to an excess hot water, a positively charged sol of hydrated ferric oxide is formed due to the adsorption of Fe3+ ions. However, when ferric chloride is added to NaOH a negatively charged sol is obtained with adsorption of OH- ions
Fe2O3.xH2O/Fe3+ Fe2O3.xH2O/OH–
Positively charged Negatively charged - Having acquired a positive or a negative charge by selective adsorption on the surface of a colloidal particle as stated above, this layer attracts counter ions from the medium forming a second layer, as shown below.
AgI/I- K+ AgI/Ag+ I - - The combination of the two layers of opposite charges around the colloidal particle is called Helmholtz electrical double layer. According to modern views, the first layer of ions is firmly held and is termed the fixed layer while the second layer is mobile which is termed diffused layer.
- Since the separation of charge is a seat of potential, the charges of opposite signs on the fixed and diffused parts of the double layer result in a difference in potential between these layers. This potential difference between the fixed layer and the diffused layer of opposite charges is called the electrokinetic potential or zeta potential.
- The presence of equal and similar charges on colloidal particles is largely responsible in providing stability to the colloidal solution because the repulsive forces between charged particles having the same charge prevent them from coalescing or aggregating when they come closer to one another.
6. Electrophoresis
The existence of charge on colloidal particles is confirmed by the electrophoresis experiment. When an electric potential is applied across two platinum electrodes dipping in a colloidal solution, the colloidal particles move towards one or the other electrode.
- The movement of colloidal particles under an applied electric potential is called electrophoresis. Positively charged particles move towards the cathode while negatively charged particles move towards the anode. This can be demonstrated by the following experimental setup (Fig.).
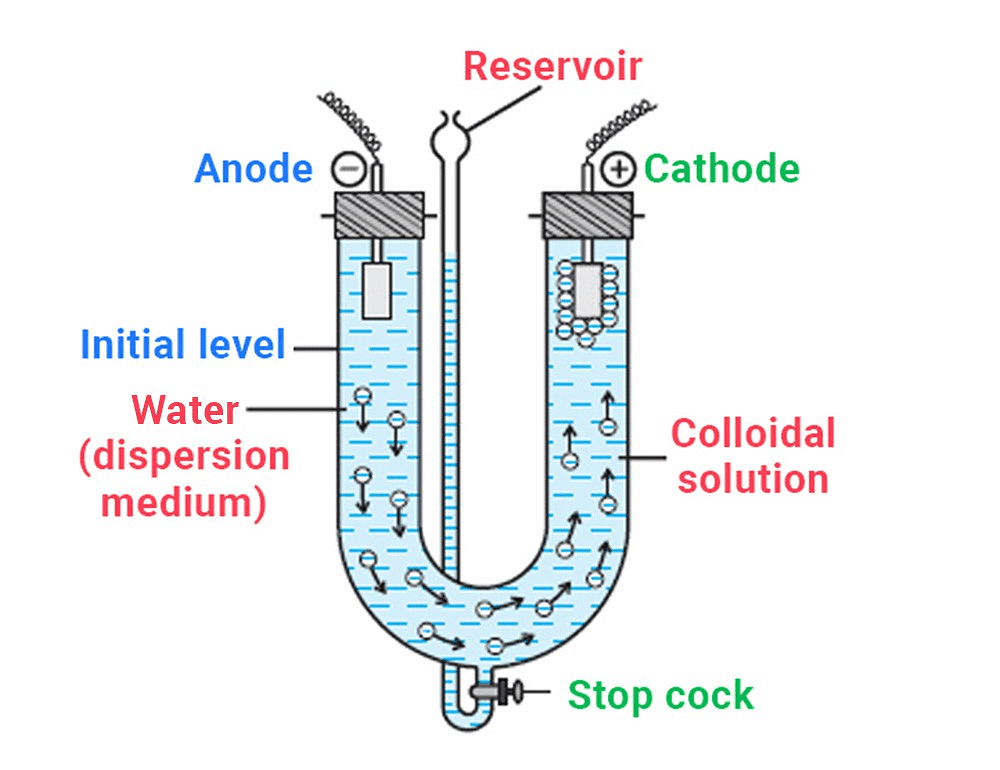
- When electrophoresis, i.e., movement of particles is prevented by some suitable means, it is observed that the dispersion medium begins to move in an electric field. This phenomenon is termed electroosmosis.
7. Coagulation or Precipitation
The stability of the lyophobic sols is due to the presence of charge on colloidal particles. If somehow, the charge is removed, the particles will come nearer to each other to form aggregates (or coagulate) and settle down under the force of gravity. The process of settling colloidal particles is called coagulation or precipitation of the sol.
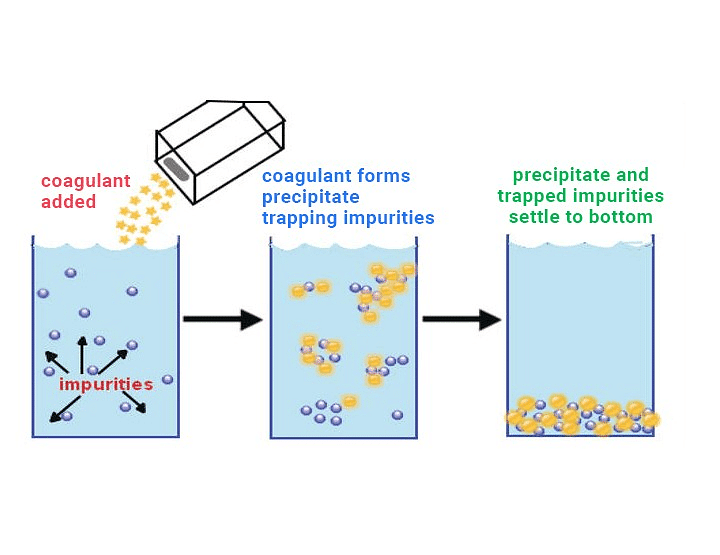
The coagulation of the lyophobic sols can be carried out in the following ways:
(i) By electrophoresis: The colloidal particles move towards oppositely charged electrodes, get discharged and precipitated.
(ii) By mixing two oppositely charged sols: Oppositely charged sols when mixed in almost equal proportions, neutralise their charges and get partially or completely precipitated. Mixing of hydrated ferric oxide (+ve sol) and arsenious sulphide (–ve sol) bring them in the precipitated forms. This type of coagulation is called mutual coagulation.
(iii) By boiling: When a sol is boiled, the adsorbed layer is disturbed due to increased collisions with the molecules of the dispersion medium. This reduces the charge on the particles and ultimately lead to settling down in the form of a precipitate.
(iv) By persistent dialysis: On prolonged dialysis, traces of the electrolyte present in the sol are removed almost completely and the colloids become unstable and ultimately coagulate.
(v) By the addition of electrolytes: When an excess of an electrolyte is added, the colloidal particles are precipitated. The reason is that colloids interact with ions carrying charge opposite to that present on themselves. This causes neutralisation leading to their coagulation. The ion responsible for the neutralisation of charge on the particles is called the coagulating ion. A negative ion causes the precipitation of positively charged sol and vice versa.
It has been observed that, generally, the greater the valence of the flocculating ion added, the greater is its power to cause precipitation. This is known as the Hardy-Schulze rule. In the coagulation of a negative sol, the flocculating power is in the order: Al3+>Ba2+>Na+
Similarly, in the coagulation of a positive sol, the flocculating power is in the order: [Fe(CN)6] 4– > PO4 3– > SO4 2– > Cl–
The minimum concentration of an electrolyte in millimoles per litre required to cause precipitation of a sol in two hours is called coagulating value. The smaller the quantity needed, the higher will be the coagulating power of an ion.
Application of Colloids

- Medicines : The medicines containing gold, silver or calcium etc. in colloidal form are more effective and easily assimilated by the human systems.
- Dyes : In dyeing, mordants colloidal substances are used in textile dyeing industry to fasten dyes.
- Rubber industry : Latex is a colloidal solution of negatively charged particles. The article to be rubber plated is made the anode. Under the influence of electric field the rubber particles get deposited on the anode and the article gets rubber plated.
- Smoke screens : Smoke screens which consist of titanium dioxide dispersed in air are used in warfare for the purpose of concealment and camouflage.
- Formation of delta : The river waver carries with it charged clay particles and many other substances in the form of colloidal solution. When the sea water comes in contact with these particles, the colloidal particles in river water are coagulated by the electrolytes present in sea water to form deltas.
- Purification of water : The turbidity in water is due to the presence of negatively charged clay particles. The addition of potash alum, i.e., Al3 ions neutralizes the negative charge on the colloidal particles and thus causes their coagulation. The coagulated matter settles down and thus becomes clear.
- Artificial rain : Artificial rain can be caused by throwing electrified sand on clouds which are colloidal solutions or charged particles of water in air.
- Smoke precipitation : Smoke coming out of the chimney is industrial area is a nuisance and health hazard. It is a colloidal particles are charged particles and thus they are removed from fuel gases by electrical precipitation (Cottrell Precipitator). In, the smoke is made to pass through chambers fitted with highly electrically charged plates which precipitate the carbon and dust particles leaving in the gases to escape through chimney (figure).
- Sewage disposal : Sewage water consists of particles of dirt, rubbish, mud, etc., which are of colloidal dimensions and carry an electric charge and thus do not settle down easily. These particles can thus be removed by cataphoresis. A system of two tanks fitted with metallic electrodes is used for this purpose. When electric field is created, then the dust particles are coagulated on he oppositely charged electrodes. The deposit may be utilized as a manure.
- Cleansing action of soap and detergent : Soap solution may be used to wash off the dirt sticking to the fabric, in the presence
it forms a collodial solution in water forms (miscelles), removes dirt by simple adsorption of oily substance and thus washes away. It decreases the interfacial tension between water and grease, and it causes the emulsification of grease in water. By mechanical action such as rubbing, the dirt particles are also detached along with the only material. - In Photography : Various colloidal system are used in photographic process. In the preparation of photographic plates, the silver bromide in gelatin is coated on thin glass plates. In developing and fixation, various colloidal substances are used. In different kinds of colour printing, gelatin and other colloidal mixtures are used.
- Blue colour of the sky : Colloidal particles scatter only blue light and the rest of is absorbed. In sky there are a number of dust and water particles. They scatter blue light and, therefore, sky looks bluish. If there were no scattering, the sky would have appeared totally dark.
|
108 videos|286 docs|123 tests
|
FAQs on Preparation, Purification & Properties of Colloidal Solutions - Chemistry Class 12 - NEET
| 1. How can colloidal solutions be purified? |  |
| 2. What are some properties of colloidal solutions? |  |
| 3. What are some common applications of colloids? |  |
| 4. How can colloidal solutions be prepared? |  |
| 5. What are some key properties of colloidal solutions that make them unique? |  |

|
Explore Courses for NEET exam
|

|