Class 10 Exam > Class 10 Notes > Chemistry for GCSE/IGCSE > Preparation of Soluble Salts
Preparation of Soluble Salts | Chemistry for GCSE/IGCSE - Class 10 PDF Download
Preparing Soluble Salts
Salts
- A salt is a compound that is formed when the hydrogen atom in an acid is replaced by a metal
- For example, if we replace the hydrogen atom in Hydrochloric Acid (HCl) with a potassium atom, then the salt potassium chloride (KCl) is formed.
- Salts play a crucial role in various applications such as fertilizers, batteries, cleaning products, healthcare items, and fungicides.
Naming salts
- Salts are compounds produced by replacing the hydrogen atom in an acid with a metal.
- When the hydrogen in an acid like Hydrochloric Acid (HCl) is substituted with a metal such as potassium, a salt like potassium chloride (KCl) is created.
- The significance of salts lies in their diverse applications spanning fertilizers, batteries, cleaning agents, medicinal products, and fungicides.
- The name of a salt comprises two parts:
- The first part is derived from the metal, metal oxide, or metal carbonate involved in the reaction.
- The second part originates from the acid used in the reaction.
- The name of a salt is determined by the reactants involved.
- For instance, when hydrochloric acid reacts, salts ending in chloride are produced, containing the chloride ion (Cl -).
- Examples:
- When sodium hydroxide reacts with hydrochloric acid, sodium chloride is produced.
- When zinc oxide reacts with sulfuric acid, zinc sulfate is produced.
Preparing Salts
- Certain salts are obtainable through mining, while others necessitate laboratory synthesis.
- The selection of method is contingent upon the solubility characteristics of the salt undergoing preparation.
Question for Preparation of Soluble SaltsTry yourself: Which of the following is true about salts?View Solution
Preparing Soluble Salts
Method A: adding acid to a solid metal, insoluble base or insoluble carbonate
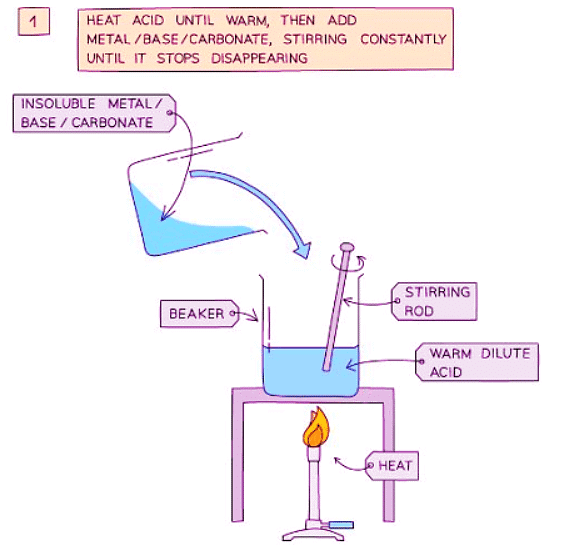
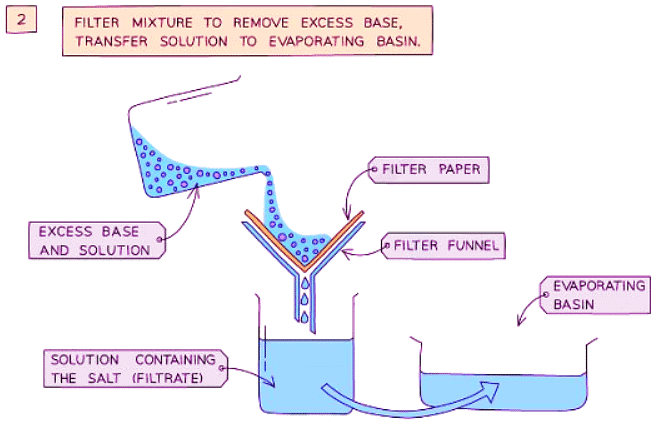
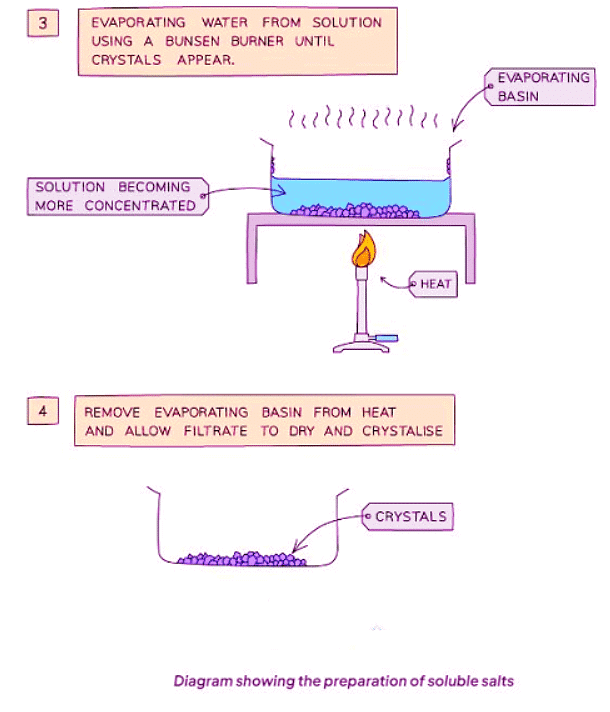
Method
- Add dilute acid into a beaker and heat using a Bunsen burner flame.
- Add the insoluble metal, base, or carbonate gradually to the warm dilute acid and stir until the base is in excess (i.e., until a suspension of the base forms in the acid).
- Filter the mixture into an evaporating basin to eliminate the excess base.
- Heat the solution to evaporate water and achieve saturation. Verify saturation by observing crystal formation on a cold, glass rod dipped into the solution.
- Allow the filtrate to dry and crystallize in a warm location.
- Remove excess solution and either air-dry the crystals or use filter paper to blot them dry.
Example: preparation of pure, hydrated copper(II) sulfate crystals using method A
Acid: Dilute sulfuric acid
Insoluble base: Copper(II) oxide
Method
- Add dilute sulfuric acid into a beaker and heat using a bunsen burner flame
- Add copper(II) oxide (insoluble base), a little at a time to the warm dilute sulfuric acid and stir until the copper (II) oxide is in excess (stops disappearing)
- Filter the mixture into an evaporating basin to remove the excess copper(II) oxide
- Leave the filtrate in a warm place to dry and crystallize
- Decant excess solution
- Blot crystals dry with filter paper
Equation of reaction: Copper(II) oxide + Sulfuric acid → Copper(II) sulphate + Water
CuO (s) + H2SO4 (aq) → CuSO4 (aq) + H2O (l)
Method B: Reacting a Dilute Acid and Alkali (Soluble Base)
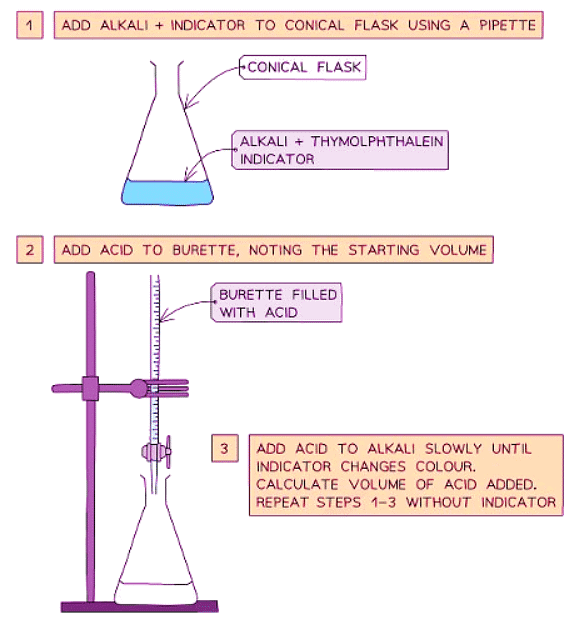
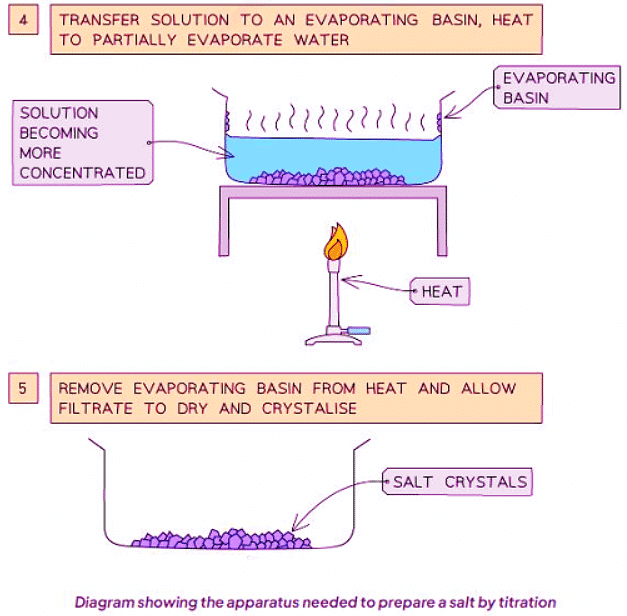
Method
- Use a pipette to measure the alkali into a conical flask and add a few drops of indicator (such as thymolphthalein or methyl orange).
- Add the acid into the burette.
- Record the starting volume of acid in the burette.
- Add the acid slowly from the burette to the conical flask until the indicator changes color appropriately.
- Record the final volume of acid in the burette.
- Calculate the volume of acid added by subtracting the initial volume from the final volume.
After determining the volume of acid needed, follow these steps:
- Add the calculated volume of acid to the same volume of alkali (without the indicator).
- Heat the resulting solution in an evaporating basin to partially evaporate, leaving a saturated solution (where crystals start to form).
- Allow the solution to cool and crystals to form, then decant the excess solution to obtain the crystals.
Steps for Preparing Soluble Salts
- Calculate the final volume of acid by subtracting the initial volume of acid from it.
- Add the obtained volume of acid to an equivalent volume of alkali without adding any indicator.
- Heat the resulting solution in an evaporating basin until partial evaporation occurs, leaving a saturated solution (visible by crystals forming on the basin's sides or on a glass rod).
- Allow the solution to cool and crystallize, then carefully decant the excess liquid and let the crystals dry.
Question for Preparation of Soluble SaltsTry yourself: What is the purpose of heating the solution in Method A when preparing soluble salts?View Solution
The document Preparation of Soluble Salts | Chemistry for GCSE/IGCSE - Class 10 is a part of the Class 10 Course Chemistry for GCSE/IGCSE.
All you need of Class 10 at this link: Class 10
|
71 videos|147 docs|61 tests
|
FAQs on Preparation of Soluble Salts - Chemistry for GCSE/IGCSE - Class 10
| 1. How can soluble salts be prepared? |  |
Ans. Soluble salts can be prepared by reacting an acid with a base, a metal, or a carbonate.
| 2. What are some common methods for preparing soluble salts? |  |
Ans. Some common methods for preparing soluble salts include precipitation, neutralization, and direct combination of elements.
| 3. Can soluble salts be prepared by mixing two soluble salts together? |  |
Ans. Yes, soluble salts can be prepared by mixing two soluble salts together through double displacement reactions.
| 4. Why is it important to ensure that the salt formed is soluble in the preparation of soluble salts? |  |
Ans. It is important to ensure that the salt formed is soluble to avoid any insoluble precipitates from forming, which could affect the purity of the final product.
| 5. What are some factors to consider when preparing soluble salts? |  |
Ans. Factors to consider when preparing soluble salts include the reactivity of the reactants, the solubility of the salt formed, and the method of preparation chosen.
Related Searches
















