NEET Previous Year Questions (2014-2024): Biomolecules | Chemistry Class 12 PDF Download
2024
Q1: The reagents with which glucose does not react to give the corresponding tests/products areA. Tollen's reagent
B. Schiff's reagent
C. HCN
D. NH2OH
E. NaHSO3
Choose the correct options from the given below:
(a) B and C
(b) A and D
(c) B and E
(d) E and D (NEET 2024)
Ans: (c)
Let's analyze each reagent and its reaction with glucose to determine which ones do not react accordingly.
Glucose is an aldohexose, meaning it has an aldehyde group in its straight-chain form and can form a cyclic hemiacetal structure. This structure is predominant under physiological conditions.
A. Tollen's Reagent: Tollen's reagent is a solution of silver nitrate in ammonia, used to detect reducing sugars. Glucose, having a free aldehyde group or an easily openable hemiacetal in its cyclic form, can reduce Tollen's reagent to metallic silver, showing a positive test. Thus, glucose reacts with Tollen's reagent.
B. Schiff's Reagent: Schiff's reagent is used to detect aldehydic groups. It is sensitive to free aldehydes, turning pink when aldehydes are present. Since the aldehyde form of glucose is present in very minute amounts at equilibrium (most glucose is in the cyclic form), it generally does not react strongly with Schiff's reagent, leading to a negative test.
C. HCN: Hydrogen cyanide (HCN) can react with carbonyl groups to form cyanohydrins. In the case of glucose, the reactive aldehyde group can react with HCN to form glucosyl cyanohydrin. Thus, glucose does react with HCN.
D. NH2OH (Hydroxylamine): Hydroxylamine reacts with carbonyl compounds to form oximes. Glucose, containing an aldehyde group, can react with hydroxylamine to form glucose oxime. Therefore, glucose reacts with hydroxylamine.
E. NaHSO3 (Sodium Bisulfite): Sodium bisulfite reacts with aldehydes to form bisulfite adducts. However, because glucose in solution predominantly exists in its cyclic form rather than the open-chain form, it does not readily react with sodium bisulfite. Hence, glucose generally does not react with sodium bisulfite, unless placed under certain conditions that favor the open-chain form.
In conclusion, the reagents with which glucose does not react to give the corresponding tests/products are:
B. Schiff's Reagent and
E. NaHSO3
Therefore, the correct answer is Option C: B and E.
2023
Q1: Given below are two statements: (NEET 2023)Statement I: A unit formed by the attachment of a base to 1' position of sugar is known as nucleoside.
Statement II: When nucleoside is linked to phosphorous acid at 5' -position of sugar moiety, we get nucleotide.
In the light of the above statements, choose the correct answer from the options given below:
(a) Both Statement I and Statement II are true
(b) Both Statement I and Statement II are false
(c) Statement I is true but Statement II is false
(d) Statement I is false but Statement II is true
Ans: (c)
 Base link with 1' position of sugar in nucleoside so statement I is correct.
Base link with 1' position of sugar in nucleoside so statement I is correct.
When nucleoside is linked to phosphoric acid at 5' position of sugar moiety we get a nucleotide
Statement II is incorrect because not link with phosphorous acid.
2022
Q1: The incorrect statement about denaturation of proteins is : (NEET 2022)
(a) Uncoiling of the helical structure takes place
(b) It results due to change of temperature and/or pH
(c) It results in loss of biological activity of proteins
(d) A protein is formed from amino acids linked by peptide bonds
Ans: (d)
- Proteins are polymers of α-amino acids and they are connected to each other by peptide bond, but this is not denaturation process.
- Due to denaturation globules unfold and helix get uncoiled and protein loses its biological activity.
- Denaturation can be caused if protein in its native form, is subjected to physical change like change in temperature or chemical change like change in pH
Q2: The incorrect statement regarding enzymes is (NEET 2022)
(a) Enzymes are biocatalysts.
(b) Like chemical catalysts enzymes reduce the activation energy of bio processes.
(c) Enzymes are polysaccharides.
(d) Enzymes are very specific for a particular reaction and substrate.
Ans: (c)
Enzymes are complex nitrogenous organic compounds which are produced by living plants and animals. They are protein molecules of high molecular mass. They are not polysaccharides.
2021
Q1: Given below are two statements :
Statement I : Aspirin and Paracetamol belong to the class of narcotic analgesics.
Statement II : Morphine and Heroin are non-narcotic analgesics.
In the light of the above statements, choose the correct answer from the options given below.
(a) Statement I is incorrect but Statement II is true.
(b) Both Statement I and Statement II are true
(c) Both Statement I and Statement II are false
(d) Statement I is correct but Statement II is false (NEET 2021)
Ans: (c)
- Aspirin and paracetamol belong to the class of non-narcotic analgesics.
- Morphine and Heroin are narcotic analgesics.
∴ Both statement I and Statement II are false.
2020
Q1: Sucrose on hydrolysis gives: (NEET 2020)
(a) α-D-Glucose + β-D-Fructose
(b) α-D-Fructose + β-D-Fructose
(c) β-D-Glucose + α-D-Fructose
(d) α-D-Glucose + β-D-Glucose
Ans: (a)
Sucrose is a disaccharide composed of two monosaccharide units, glucose and fructose, joined together via a glycosidic bond. Upon hydrolysis, this bond is broken, and the two constituent monosaccharides are released. Hydrolysis of sucrose results in the formation of one molecule of glucose and one molecule of fructose. However, it is important to specify the configurations of these monosaccharides as they exist in specific forms.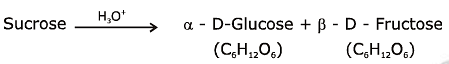
This reflects the correct stereochemistry and identity of the monosaccharides produced from the hydrolysis of sucrose.
Q2: Which of the following is a basic amino acid? (NEET 2020)
(a) Tyrosine
(b) Lysine
(c) Serine
(d) Alanine
Ans: (b)
Lysine is a basic amino acid it contains more number of − NH2 groups as compared to − COOH groups due to their symmetrical.
2019
Q1: The non-essential amino acid among the following is: (NEET 2019)
(a) Valine
(b) Leucine
(c) Alanine
(d) Lysine
Ans: (c)
Non Essential amino acid is Alanine.
2018
Q1: The difference between amylose and amylopectin is (NEET 2018)
(a) Amylopectin have 1 → 4 α-linkage and 1→ 6α-linkage
(b) Amylose have 1 → 4 α-linkage and 1→ 6β-linkage
(c) Amylopectin have 1 → 4 α-linkage and 1→ 6β-linkage
(d) Amylose is made up of glucose and galactose
Ans: (a)
Amylose is long unbranched chain with α-D-Glucose with held by C1–C4 glucosidic linkage whereas amylopectin is branched chain polymer of α-D glucose unit in which chain is formed by C1–C4 glycosidic linkage while branching occurs by C1–C6 glucosidic linkage.
Q2: Which of the following compounds can form a zwitterion ? (NEET 2018)
(a) Aniline
(b) Acetanilide
(c) Benzoic acid
(d) Glycine
Ans: (d)
2017
Q.8. Which of the following statements is not correct :- (NEET 2017)
(a) Ovalbumin is a simple food reserve in egg-white
(b) Blood proteins thrombin and fibrinogen are involved in blood clotting
(c) Denaturation makes the proteins more active
(d) Insulin maintanis sugar level in the blood of a human body
Ans: (c)
Denaturation changes the structure of a protein and protein loses its activity.
2016
Q1: In a protein molecule various amino acids are linked together by: (NEET 2016 Phase 1)
(a) dative bond
(b) α-glycosidic bond
(c) β-glycosidic bond
(d) peptide bond
Ans: (d)
In a protein molecule various amino acids are linked together by peptide bond.
Q2: Which one given below is a non-reducing sugar ? (NEET 2016 Phase 1)
(a) Sucrose
(b) Maltose
(c) Lactose
(d) Glucose
Ans: (a)
All monosaccharides whether aldoses or ketoses are reducing sugars. Disaccharides such as sucrose in which the two monosaccharide units are linked through their reducing centres i.e., aldehydic or ketonic groups are non-reducing.
Q3: The correct statement regarding RNA and DNA, respectively is: (NEET 2016 Phase 1)
(a) The sugar component in RNA is 2'-deoxyribose and the sugar component in DNA is arabinose.
(b) The sugar component in RNA is arabinose and the sugar component in DNA is 2'-deoxyribose.
(c) The sugar component in RNA is ribose and the sugar component in DNA is 2'-deoxyribose.
(d) The sugar component in RNA is arabinose and the sugar component in DNA is ribose.
Ans: (c)
Sugar in DNA is 2'-deoxyribose whereas sugar in RNA is ribose.
Q4: The central dogma of molecular genetics states that the genetic information flows from
(a) Amino acids
(b) DNA
(c) DNA
(d) DNA
Ans: (c)
The central dogma of molecular genetics is

Q5: The correct corresponding order of names of four aldoses with configuration given below

(b) D-threose, D-erythrose, L-threose, L-erythrose
(c) L-erythrose, L-threose, D-erythrose, D-threose
(d) D-erythrose, D-threose. L-erythrose, L-threose (NEET 2016 Phase 2)
Ans: (d)

2014
Q1: Which of the following hormones is produced under the condition of stress which stimulates glycogenolysis in the liver of human beings ? (NEET 2014)
(a) Adrenaline
(b) Estradiol
(c) Thyroxin
(d) Insulin
Ans: (a)
Adrenaline is a hormone produced by adrenal glands during high stress or exciting situations. This powerful hormone is part of the human body’s acute stress response system, also called the fight or flight response
Q2: D(+) glucose reacts with hydroxyl amine and yields an oxime. The structure of the oxime would be: (NEET 2014)
(a)

(b)

(c)
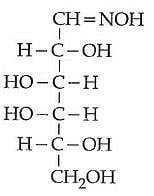
(d)
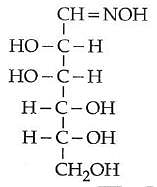
Ans: (b)
|
108 videos|286 docs|123 tests
|
FAQs on NEET Previous Year Questions (2014-2024): Biomolecules - Chemistry Class 12
| 1. What are biomolecules? |  |
| 2. What is the importance of biomolecules in living organisms? |  |
| 3. How are biomolecules classified? |  |
| 4. What are some examples of biomolecules? |  |
| 5. How do biomolecules contribute to disease development? |  |

|
Explore Courses for NEET exam
|

|


















