NEET Previous Year Questions (2014-2024): Some Basic Concepts of Chemistry | Chemistry Class 11 PDF Download
Q.1. The highest number of helium atoms is in (2024)
(a) 4mol of helium
(b) 4u of helium
(c) 4g of helium
(d) 2.271098L of helium at STP
Ans: (a)
To determine which option contains the highest number of helium atoms, we need to analyze each option based on the amount of helium it represents and apply Avogadro's Law as required.
Option A: 4mol of helium
Using Avogadro's number, which is approximately 6.022 × 1023 atoms per mole, the number of helium atoms in 4 moles can be calculated as: 4mol × 6.022 × 1023 atoms/mol = 24.088 × 1023 atoms
Option B: 4u of helium
The atomic mass of helium is approximately 4 u (atomic mass units). Therefore, 4u represents about 1 mole of helium atoms (since the molar mass of helium is approximately 4 g/mol, which equals 4 u). Thus, this option represents:
1mol × 6.022 × 1023 atoms/mol = 6.022 × 1023 atoms
Option C: 4g of helium
Similarly, as we've established that the molar mass of helium is 4 g/mol, 4g of helium equates exactly to:
1mol × 6.022 × 1023 atoms/mol = 6.022 × 1023 atoms
Option D: 2.271098L of helium at STP (Standard Temperature and Pressure)
At STP, one mole of any ideal gas occupies 22.4 L. Therefore, the amount of helium in moles for L
2.271098L can be derived from:

Using this to find the number of atoms:
0.101mol × 6.022 × 1023 atoms/mol ≈ 6.082 × 1022 atoms
Conclusion:
Comparing the numbers:
Option A: 24.088 × 1023 atoms
Option B: 6.022 × 1023 atoms
Option C: 6.022 × 1023 atoms
Option D: 6.082 × 1022 atoms
Option A clearly contains the highest number of helium atoms, which is 24.088 × 1023 atoms
Q2: 1 gram of sodium hydroxide was treated with 25mL of 0.75M HCl solution, the mass of sodium hydroxide left unreacted is equal to
(a) 750 mg
(b) 250 mg
(c) Zero mg
(d) 200 mg (2024)
Ans: (b)
To find the mass of sodium hydroxide (NaOH) left unreacted, we first need to determine the moles of NaOH and HCl originally present and compare them to see which one is in excess.
The molar mass of NaOH is approximately 40 g/mol, so the number of moles of NaOH in 1 gram can be calculated as follows: Next, we calculate the number of moles of HCl using its concentration and the volume of the solution. Recall that concentration (Molarity, M) is defined as moles of solute per liter of solution. Given that the concentration of HCl is 0.75 M and the volume of the solution is 25 mL or 0.025L, we can find the moles of HCl:
Next, we calculate the number of moles of HCl using its concentration and the volume of the solution. Recall that concentration (Molarity, M) is defined as moles of solute per liter of solution. Given that the concentration of HCl is 0.75 M and the volume of the solution is 25 mL or 0.025L, we can find the moles of HCl:
Moles of HCl = Concentration × Volume in liters = 0.75 M × 0.025 L = 0.01875 moles
Now, we compare the moles of NaOH and HCl. The stoichiometry of the reaction between NaOH and HCl is:
NaOH + HCl → NaCl + H2O
Each mole of NaOH reacts with one mole of HCl. Given that there are more moles of NaOH (0.025 moles) than HCl (0.01875 moles), HCl is the limiting reactant and will be completely consumed. The excess NaOH can be calculated:
Excess moles of NaOH = Moles of NaOH − Moles of HCl = 0.025 moles − 0.01875 moles = 0.00625 moles
To find the mass of the unreacted NaOH:
Mass of unreacted NaOH = Moles of unreacted NaOH × Molar mass of NaOH = 0.00625 moles × 40 g/mol = 0.25 g = 250 mg
Therefore, the mass of sodium hydroxide left unreacted is 250 mg, which corresponds to Option B.
Q.3. A compound X contains 32% of A, 20% of B and remaining percentage of C. Then, the empirical formula of X is :
(Given atomic masses of A = 64 ; B=40 ; C = 32 u)
(a) A2BC2
(b) ABC3
(c) AB2C2
(d) DABC4 (2024)
Ans: (b) So, empirical formula of
So, empirical formula of
∴ The correct empirical formula of compound X is ABC3
Q.4. A compound with a molecular formula of C6H14 has two tertiary carbons. Its IUPAC name is :
(a) n-hexane
(b) 2-methylpentane
(c) 2,3-dimethylbutane
(d) 2,2-dimethylbutane (2024)
Ans: (c)
Q.5. Select the correct statements from the following (2023)
A. Atoms of all elements are composed of two fundamental particles.
B. The mass of the electron is 9.10939 × 10–31 kg.
C. All the isotopes of a given element show same chemical properties.
D. Protons and electrons are collectively known as nucleons.
E. Dalton’s atomic theory, regarded the atom as an ultimate particle of matter.
Choose the correct answer from the options given below
(a) A, B and C only
(b) C, D and E only
(c) A and E only
(d) B, C and E only
Ans: d
- Atoms consist of three fundamental particles: Electrons, protons and neutrons
- The mass of the electron is 9.10939 × 10–31 kg
- All the isotopes of a given element show same chemical properties.
- Protons and neutrons present in the nucleus are collectively called as nucleons.
- Dalton’s atomic theory, regarded the atom as the ultimate particle of matter.
So, the correct statements are B, C, E only

Q.6. What mass of 95% pure CaCO3 will be required to neutralise 50 mL of 0.5 M HCl solution according to the following reactions?
[Calculate upto second place of decimal point] (2022)
(a) 3.65 g
(b) 9.50 g
(c) 1.25 g
(d) 1.32 g
Ans: d
Q.7. An organic compound contains 80% (by wt.) carbon and the remaining percentage of hydrogen. The right option for the empirical formula of this compound is: [Atomic wt. of C is 12, H is 1] (2021)
(a) CH3
(b) CH4
(c) CH
(d) CH2
Ans: a
An empirical formula represents the simplest whole number ratio of various atoms present in a compound, whereas, the molecular formula shows the exact number of different types of atoms present in a molecule of a compound.
Q.8. Which one of the followings has maximum number of atoms? (2020)
(a) 1g of O2(g) (Atomic mass of O = 16)
(b) 1g of Li(s) (Atomic mass of Li = 7)
(c) 1g of Ag(s) (Atomic mass of Ag = 108)
(d) 1g of Mg(s) (Atomic mass of Mg = 24)
Ans: b
(a) no. of atoms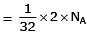
(b) no. of atoms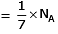
(c) no. of atoms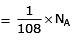
(d) no. of atoms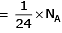
∴ 1 gm of Li is having maximum no. of atoms.
Q.9. The number of moles of hydrogen molecules required to produce 20 moles of ammonia through Haber's process is : (2019)
(a) 10
(b) 20
(c) 30
(d) 40
Ans: c
Haber's process
20 moles need to be produced
2 moles of NH3 → 3 moles of H2
Hence 20 moles of  moles of H2
moles of H2
Q.10. In which case is the number of molecules of water maximum? (2018)
(a) 18 mL of water
(b) 0.18 g of water
(c) 0.00224 L of water vapours at 1 atm and 273 K
(d) 10-3 mol of water
Ans: a

Q.11. A mixture of gases contains H2 and O2 gases in the ratio of 1 : 4 (w/w). What is the molar ratio of the two gases in the mixture ? (2015)
(a) 2:1
(b) 1:4
(c) 4:1
(d) 16:1
Ans: c
(At. wt. Mg = 24; O = 16)
Q.12. When 22.4 litres of H2 (g) is mixed with 11.2 litres of Cl2 (g) , each at S.T.P., the moles of HCl (g) formed is equal to : (2014)
(a) 0.5 mol of HCl (g)
(b) 1.5 mol of HCl (g)
(c) 1 mol of HCl (g)
(d) 2 mol of HCl (g)
Ans: c
1 mole = 22.4 liters at S.T.P.
Here, Cl2 is limiting reagent. So, 1 mole of HCI(g) is formed.
|
127 videos|244 docs|87 tests
|
FAQs on NEET Previous Year Questions (2014-2024): Some Basic Concepts of Chemistry - Chemistry Class 11
| 1. What are the basic concepts of chemistry? |  |
| 2. How are elements and compounds different in chemistry? |  |
| 3. What is the importance of understanding atoms and molecules in chemistry? |  |
| 4. How does the periodic table help in organizing elements in chemistry? |  |
| 5. What role do chemical reactions play in chemistry? |  |

|
Explore Courses for NEET exam
|

|






















